Contents
Everything you need to know about Fluxes and Mineralizers in
Clinkering Process
[wpecpp name=”package” price=”75″ align=”center”]
by Vagn Johansen* and Javed I. Bhatty**
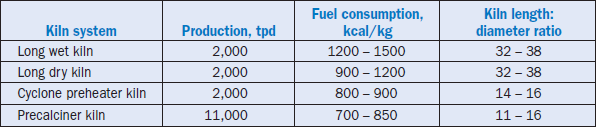
Cement manufacturing is an energy intensive process. About 80% of the total energy required in cement manufacturing is consumed in the thermal conversion of raw feed into clinker. Typically, a long dry process kiln consumes 5.0 GJ/t (1200 kcal/kg) of energy to produce clinker, compared to 5.88 GJ/t (1400 kcal/kg) for the wet process, and 3.78 GJ/t (900 kcal/kg) for the suspension preheater processes. The advent of preheater/ precalciner technology in modern plants has further reduced the heat consumption to 2.9 GJ/t (700 kcal/kg) depending on the number of preheater stages. Table 3.5.1 shows typical data for production and specific fuel consumption of different kiln systems (Alsop and others, 2001).

Figure 3.5.1. View of the Ålborg Portland cement plant in Denmark showing the preheater tower and rotary kiln for production of mineralized clinker. (Photo courtesy Duncan Herfort, Ålborg Portland).
Table 3.5.1. Typical Data for Various Kiln Systems (After Alsop and Others, 2001)
HISTORICAL BACKGROUND
Comprehensive literature reviews on the use of fluxes and mineralizers have been made by Klemm and Skalny (1976), Viswanatha and Ghosh (1980), Mishulovich (1994), and Bhatty (1996) covering numerous references from 1875 to 1995. As pointed out by Bhatty (1996), although promising results have been reported on the use of fluxes and mineralizers, not much has been implemented in practice. References made to fluorine compounds are considerably larger in number than to any other compounds used as mineralizers. Kuehl (1952), and Klemm and Skalny (1976) noted that the earliest reports on the use of fluorspar (CaF2) in clinker burning were by Michaelis (1875) and Erdmenger (1882).
Practical experience in the 1930s was expressed in F.L. Smidth & Co’s manuals for traveling kiln start-up personnel where the use of fluorspar was mentioned as helpful in cases when clinker were difficult to burn and/or there were problems with soundness of the cement (Osbaeck 2000). Flint (1939) mentioned magnesium silicofluoride as a good mineralizer for production of white cement clinker. Lately, based on the development work by Blue Circle (Moir 1982), the so-called mineral-ized clinker using controlled mixtures of fluorspar and gypsum added to the kiln feed, is being manufactured in Denmark and other countries (Borgholm and others, 1995; Borgholm, 1996). Ålborg Portland cement plant in Denmark (Figure 3.5.1) is an example of industrial clinker production using mineralizers. So it seems that fluorspar was the first mineralizer to be used, and it is still in use, whereas the other compounds reported in the literature are rarely used when it comes to industrial application – as Bhatty (1996) already had indicated.
Modern kiln systems are very compact and the heat losses due to radiation and convection are rela-tively small. Decrease in the burning zone temperature may have a marginal effect on these losses. However, this temperature has to be high enough for sufficient amount of clinker melt to form. The function of the clinker melt is two fold: 1) it controls the agglomeration and cohesion of the mate-rial in the burning zone of the rotary kiln, and thereby the mass flow through the kiln, and 2) it controls the rate of clinker mineral formation specifically the consumption of calcium oxide and formation of alite, by acting as the transport medium for the reactants. An example of the role of the liquid phase is the case of white cement clinker. The amount of liquid phase in white cement is usually lower than in corresponding gray cement clinker; also the liquid is formed at higher temper-atures. As a result it is more difficult to burn the mix and this is evidenced by the practical experi-ence that the production of white cement clinker is about 70% of the production of gray clinker on a kiln of similar physical size; the specific fuel consumption is correspondingly higher.
However, any potential for energy saving by reducing the clinkering temperature and promoting the reaction rates appears promising. Several options have been evaluated to reduce the thermal demand in cement manufacturing without adversely affecting the clinker quality. As noted by Klemm and Skalny (1976), improvement in the rate of clinker formation, or the burnability of the kiln feed, can be brought about in several ways, 1) by substantially decreasing particle size of the raw mix, 2) by introducing a few natural raw materials of unusually high homogeneity and favorable chemical composition and, 3) by using fluxing agents or mineralizers to lower the melting point of the clinker liquid phase – all means of increasing the reaction rates at lower temperatures. Fluxes and/or mineralizers have impact on pyroprocessing and operational advantages such as longer refractory life and reduced cooler maintenance.
The work described in the literature reveals great variety in experimental conditions such as clinker composition, little or no melt phase during laboratory burning experiments, many differ-ent burning temperatures, and residence times. This makes comparison among different fluxes and mineralizers difficult. The following is a review of the role of fluxes and mineralizers in clinker burning and clinker formation with respect to practical experience and reaction kinetics, with emphasis on the reaction CaO + C2S → C3S in presence of melt phase.
DEFINITIONS
Fluxes
According to Klemm and Skalny (1976), “a flux is a substance which decreases the melting point of the liquid phase, whereas a mineralizer is a substance that accelerates the rate of process or reac-tion occurring in the solid state within the liquid phase, or at the liquid-solid interface.” Taylor (1997) used a more general definition stating that, “a flux is a material that promotes the clinkering reaction by increasing the quantity of liquid at a given temperature.” Viswanathan and Ghosh (1983) used a similar definition, “fluxes are generally considered as materials that lower the temperature of liquid formation, while mineralizers accelerate the kinetics of reaction through modification of solid- and liquid-state sintering.”
From this the definition of fluxes is clear – they are materials that lower the temperature of liquid formation and/or increase the quantity of liquid at a given temperature. Addition of Al2O3 and Fe2O3-containing raw materials to a raw mix are examples of fluxes, and it is known that the burnability is improved by such changes. These additions are equivalent to a decrease in the silica ratio, SR, of the raw mix and represent an increase in liquid phase. The silica ratio is defined as SR = SiO2/ (Al2O3+Fe2O3). The liquid phase is the transport medium for the reactants in alite formation; and the more the liquid is present, the more reactants can be transported through a given cross-section in a given time, thereby increasing the rate of alite formation.
Presence of MgO also plays a critical role in melt formation. Increasing the MgO content in raw feed results in an increased amount of melt phase. However, only up to about 2% MgO by weight of raw feed functions as a flux where it lowers the melting point and contributes to the liquid formation in clinker. Addition of MgO over this amount remains as uncombined periclase and does not promote clinkering.
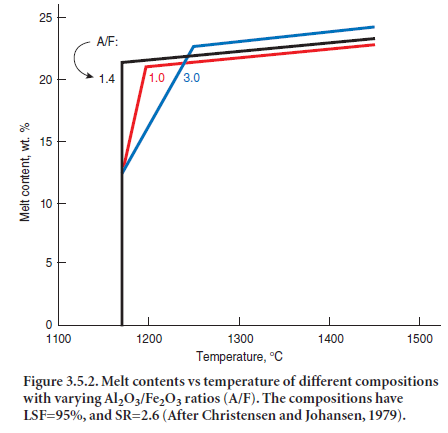
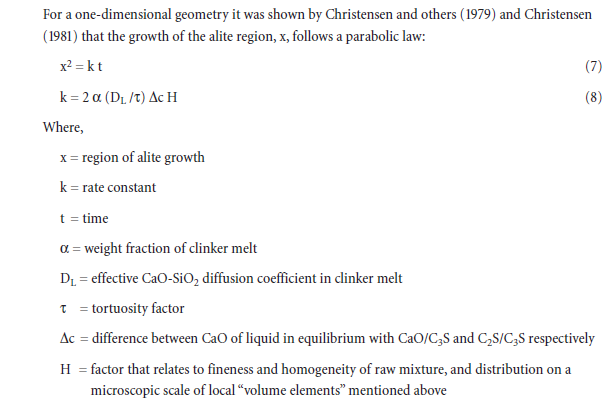
In the presence of MgO, Al2O3 and Fe2O3 or a combination of these can impart more effective flux-ing characteristics if their ratio (A/F) is within specific limits of 1.38 and 1.63 (Herath and Glasser, 1978; Bucchi, 1980; and Timashev, 1980). With MgO, the eutectic point occurs at A/F = 1.63; with-out MgO, the eutectic point occurs at A/F = 1.38. Increasing or decreasing the A/F ratio from these limits requires higher temperatures to attain the same level of melt content. Figure 3.5.2 illustrates the role of A/F ratios on melt contents.
Alkalies and sulfates also act as flux but with some qualifications (Bhatty, 1996). The use of alkali salts and sulfates requires careful selection and controlled addition. Alkali sulfates form melts at low temperatures, but such melts are immiscible with the clinker melt (Viswanatha and Ghosh, 1980) and it is questionable if it can act as a flux in the formation of alite even though it might aid in the nodulization of clinker. Alkalies not associated with sulfate can enter the clinker melt, but their effect on alite formation is not favorable (Christensen, 1980).
Mineralizers
A mineralizer accelerates the reaction rate and promotes the formation of alite, also frequently termed as C3S, within the liquid phase by lowering the stability limit of C3S below 1250°C. Fluorides and zinc compounds are mineralizers under this definition. A variety of fluoride-containing compounds such as CaF2,NaF, and MgF2 are effective mineralizers, and so are certain alkaline-and alkaline-earth fluorosilicate salts such as Na2SiF6 and MgSiF6.Erhard (1994) has reported using fluoride-based compounds as mineralizers with measurable product, operational, and energy benefits.
Some substances can function both as fluxes and mineralizers in that they increase the amount of liquid at the given temperature and decrease the stability temperature of alite (Klemm and Skalny, 1976).
CLINKER BURNING – A CHEMICAL PROCESS
The production of portland cement clinker from heterogeneous mixtures of raw materials is a complex process in which one must begin with an understanding of the principles underlying the effects of temperature, chemical composition, residence time, and process variations.
The transformation of the kiln feed to clinker in a rotary kiln is comprised of a set of unit opera-tions including:
1. Drying and dehydrating the raw material components
2. Heating the kiln feed
3. Decomposing the CaCO3
4. Agglomeration and formation of nodules
5. Synthesis of the clinker minerals
6. Cooling
Originally all these steps took place in the long wet process rotary kiln, which in a way was a unique piece of machinery with respect to combining all these unit operations. As the energy prices increased and technology improved, the kiln developed into the present day dry process consisting of, 1) preheater with a number of cyclone stages (5 or even 6 in modern kiln systems) in which the kiln feed is dried and heated in counter current with the hot exhaust gases from the kiln, and 2) preheaters in conjunction with a calciner in which the calcination process takes place. The modern short rotary kiln is now a reactor where the calcined kiln feed partially melts and the material forms nodules in which the synthesis of alite takes place under consumption of the free lime formed during the calcination process.
Much attention has been given to solid-state reactions and formation of intermediate compounds at lower temperatures in the chemical system related to portland cement. When the retention time in various parts of modern kiln systems are considered with respect to the synthesis of alite and belite, the practical importance of such possible compounds is not clear. The time for the kiln feed to fall through the preheater and pass through the calciner to the kiln inlet is typically less than 2minutes. Furthermore, during this time the kiln feed particles are suspended in the gas and only little inter-particle contact is established, and since the kiln feed in most cases is a mixture of calcareous and argillaceous materials, inter-particle contact is necessary for reaction to take place. As a matter of fact the inter-particle contact is kept to a minimum to avoid build-ups and plugging of the preheater cyclones and calciner.
For the material to travel from the kiln inlet (where material temperature is around 900°C) to the burning zone, or rather to the part of the kiln where the hard coating begins, it takes 5 to 10 minutes. The retention time in the burning zone is of the order of 45 minutes, and another 45 minutes to pass through the cooler. Furthermore, it also has to be taken into consideration that solid-solid reactions are many orders of magnitude slower than when liquid phase is involved
(Lawrence, 1997; Christensen, 1981; Viswanathan and Ghosh 1980), and except for possible minor amounts of alkali sulfate and chloride melts, liquid is present only in the burning zone. This, together with the short retention times at temperatures lower than in the burning zone, implies that solid state reactions and possible formation of intermediate products are of limited impor-tance relative to the net reaction:
CaO + belite → alite
This reaction is most critical for the consumption of CaO and the subsequent alite formation. It is the rate of this reaction that the application of fluxes and mineralizers targets. In this regard the material in the burning zone can be considered as consisting of a liquid phase (≈ 20% by weight)with various amounts of CaO, belite, and alite depending on the location in the burning zone. At the entrance, the alite content is close to zero and free CaO is high, and at the exit of the burning zone the alite content is highest and free CaO is low. Upon cooling, C3A and C4AF crystallize out of the liquid phase.
Coatings and build-ups at various locations in the kiln system may consist of intermediate compounds, and to the extent they break off and end up in the burning zone they may have some effect on the clinker produced. However, it can only be a small fraction of the total clinker produc-tion – and the aim of kiln process control is to avoid heavy builds up and their breakdowns. One point of interest is the interaction between gaseous compounds in the kiln atmosphere and the kiln feed and dust. Here the intimate contact between gas and material in preheater and calciner is very important for possible absorption of alkalis, chlorides, SO2,and CO2.However, volatiles such as alkali sulfate and chloride are present in various amounts due to internal circulation and can contribute to the formation of melt phase at temperatures lower than 1250°C at which it otherwise tends to form.
THEORETICAL CONSIDERATIONS
Role of Clinker Melt
It has already been mentioned that the melt phase plays two roles: 1) as nodulizing medium, and 2) as transport medium for the reactants involved in the chemical reactions. In the system CaO – SiO2 -Al2O3 -Fe2O3,which is a good model system for cement clinker, the liquid forms discretely at eutectic temperatures and increases only modestly in amount as the temperature is increased further. Lea and Parker (1935) gave the following formulae for the formation of liquid at various temperatures:
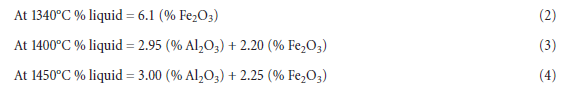
Besides, the commercial raw mixes often contain a number of minor components. For kiln feeds containing MgO and alkalies, the amount of liquid will increase by approximately the same amount as of these compounds. However, the temperature of the melt formation for such kiln feed is decreased; with MgO and alkalies the temperature is about 1280°C. Viswanathan and Ghosh(1982) made reference to work by Sorrentino and others (1975) reporting 1290°C to be considered the lowest theoretical limit for clinkerization since C3S does not form below 1250°C in the non-mineralized system.
As shown by the above equations, almost all of the melt is formed at the eutectic temperature and it increases only slightly with further increase in temperature. In other words, the clinker melt forms to nearly its full amount over a very short distance in the rotary kiln. This corresponds to where the hard coating starts at the entrance to the burning zone. At this point the material may be considered as a mixture of particles in liquid. These particles are the result of the calcining process and consist primarily of free lime and C2S. Capillary forces controlled by surface tension of the melt relative to particles result in the formation of agglomerates and nodules and in these agglomerates and nodules the reactants CaO and C2S are fixed in “local volume elements” varying in chemical composition relative to average composition of the kiln feed. The variation in local composition relative to the overall kiln feed average depends on the particle size distribution (i.e., the particle fineness) of the kiln feed and the mineralogy of the raw materials. Owing to the random distribu-tion of particles there are some “local volume elements” with high content of CaO (LSF > 100) and some with low CaO content, and vice versa with respect to SiO2 content. The rate of consumption of CaO to form C3S (i.e., the burnability) is controlled by transport of the reactants through the clinker melt between such “local volume elements.” The rate is a function of 1) difference in chem-ical composition between these “local volume elements” and 2) the quantity of clinker melt.

Surface Tension and Viscosity
It is often stated that surface tension and viscosity of the clinker melt influence the reactivity of the raw mix and formation of clinker minerals. Examples of typical values of viscosity and surface tension of melts at 1450°C as reported by Butt and others (1974), are given in Table 3.5.2; compari-son of these parameters at eutectic melt temperature is also shown in the table.
The surface tension determines how the liquid will wet the particles in the system. From observa-tions of polished sections of clinker it appears that the liquid wets the surfaces completely. It also is the force that keeps the agglomerates of material in the burning zone together and reduces poros-ity of the clinker modules formed during the burning process. The former raw material particles are distributed in the volume of the nodules and the distances over which the reactants have to be transported to complete the alite formation are fixed.
Table 3.5.2. Typical Values of Viscosity and Surface Tension of Melts at 1450°C and Eutectic Point 1338°C (Butt and Others, 1974)
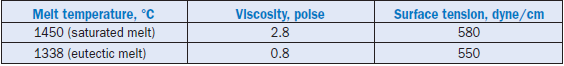
For comparison the viscosity of 97% glycerol at 20°C is 0.8 poise, and surface tension of mercury at room temperature is 472 dyne/cm.
The viscosity is a measure of the resistance to move the particles relative to each other. When the first loose agglomerates are formed, their strength is determined by the surface tension and viscoc-ity of the clinker melt. Later when these agglomerates have been compacted and have survived the tumbling movement in the rotary kiln and the clinker nodules are formed, they behave like a visco-elastic medium with an apparent viscosity 106 times larger than that of the clinker melt
(Petersen, 1983a, 1983b; Egeloev and Petersen, 1981).
Addition of minor components, which enter into the clinker melt, might change the surface energy and viscosity, and as such make nodules weaker or stronger depending on their effect on the surface energy. Weaker nodules may increase the mixing of the raw material particles in the tumbling movement in the rotary kiln; and this in turn could have an effect on the rate of forma-tion of alite from the reaction between CaO and belite. In regards to this it is worth noting that in modern kiln systems where all dust is returned, the concentration of alkali, sulfate, and chloride in the kiln can get relatively high due to the internal circulation caused by their volatility in the burn-ing zone. In practice, this means that the clinker liquid is high in concentration with respect to these minor components and their possible effects have already been realized in the clinker produced.
The literature on properties of clinker melt, viscosity and surface tension, and their possible effect on burnability of kiln feed and formation of clinker minerals, was extensively reviewed by Klemm and Skalny (1976) and Viswanathan and Ghosh (1983) in their general reviews on mineralizers and fluxes. The theme is that the viscosity of the clinker melt affects diffusion of the relevant species. Butt and others (1974) and Timashev (1980) reported a negative effect of sodium and potassium on the diffusion coefficients of the melt, resulting in simultaneous reduction in surface tension and increase in melt viscosity. Other studies reviewed by Viswanathan and Ghosh (1982) reported that MgO and SO3 also decrease the surface tension of the melt. When alkalies are in combination with SO3,a phase separation takes place and alkali sulfate melt separates out of the clinker melt (Pliego-Cuervo, 1977, 1979). This was also noted in a paper on mineralized clinker by Borgholm and others (1995). Tsuboi and co-workers (1972a, 1972b), and Timashev and Albats (1974) reported that SO3 in the raw mix or fuel rendered sintering more difficult because of the increased viscosity of the liquid phase, and the C3S crystals became much larger because of the decreased surface tension.
Klemm and Skalny (1976) also quoted systematic studies of the effect of fluxes and mineralizers on the burning of cement clinker as reported by Teoreanu (1971, 1974), Grachin and others (1971), Ponomarev and others (1966), Nagai and Tahakara (1936), Okorov and others (1957) and P’yashev and Tikhonenkova (1957). From their studies, there appears to be a direct relationship between the decrease in viscosity of the liquid phase and specific features of the mineralizer ions, such as elec-tronegativity. For cations, the influence on the reduction of liquid phase viscosity is in the order: Be2+ > Mg2+> Sr2+ > Li2+ > Ba2+ > Na1+ > K1+,which is an approximate order of their elec-tronegativities. Similarly, the lessening mineralizing effect of an anion parallels its decrease in elec-tronegativity, as in the series: SiF62- > F1- > SO42- > Cl1-.Table 3.5.3 shows the electronegativity data of different ions with their decreasing degrees of effectiveness (from left to right). A relation-ship between viscosity and the electronegativity of different elements, (Figure 3.5.3), was also reported also by Timachev (1980). The relationship exhibits a viable link between the reduction of melt viscosity and increasing electronegativity of the incorporated ions.
Not quite in accordance with this are the results of Butt and others (1974) and Timashev (1980) showing that sodium and potassium actually increase the viscosity. Shubin (1974) investigated the effect of CaF2,P2O5,Cr2O3,MnO, and MgO additives on the sintering and shrinkage of cement clinker within the temperature range of 1300°C to 1500°C. All of these additives increased the rate of sintering by 8% to 30%. The greatest effects were caused by MnO and CaF2,which was explained by a decrease in the viscosity of the liquid phase, a reduction of the temperature at which the liquid was formed, and a higher reactivity of the mix components. MgO had little effect on sintering due to an apparent increase in viscosity. However, Tsuboi and others (1972a, 1972b) found that MgO lowers the viscosity of the liquid phase and promotes the formation of small alite crystals.
Based on work by Butt and others (1974), Viswanathan and Ghosh (1982) explained the clinker melt to be as a cluster of cations and anionic groups in which the self diffusion of cations occurs by exchange of place between a vacancy and the diffusing ion. The silicate, aluminate, and ferrite anions diffuse by interchange of place or by rotation of corresponding related groups. Sodium and potassium ions affect the diffusion coefficient negatively, whereas Mg and sulfate ions increase the diffusion coefficient.
Alite Formation
The reaction under consideration is CaO + belite → alite, in the presence of clinker melt at temperatures corresponding to the burning zone in rotary kilns. It should be kept in mind that for
Table 3.5.3. Decreasing Effectiveness (from Left to Right) in the Reducing Melt Viscosity as a Function of Electronegativety of Different Ions
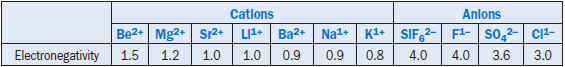
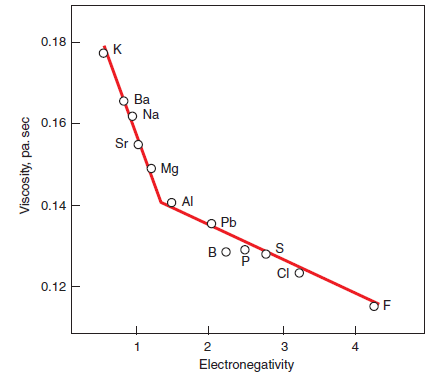
Figure 3.5.3. Correlation between melt viscosity and electronegativity of different elements
(After Timachev, 1980).
a kiln to be operated in a steady state, the temperature profile in the burning zone essentially has to correspond to high enough temperatures so that the cohesiveness of the material due to the melt content achieves a constant but relatively slow flow through the burning zone. Because, for a given composition, the clinker melt varies from a possible maximum to zero over a narrow temperature range, operating the kiln close to these temperatures becomes very critical. If the amount of melt is reduced, the material may rush through the kiln and fill up the clinker cooler causing the opera-tion to become unstable and resulting in loss of production and damage to refractory linings both in the kiln and the cooler.
Kinetics of Alite Formation
An observation from microscopical examination of clinkers is that local regions of free CaO, whether a cluster of free CaO on its own, or CaO together with alite, with few exceptions always are separated from areas with belite by regions with alite only (Chromy, 1974; Johansen, 1979).
Atypical example of such a clinker is shown by a photomicrograph in Figure 3.5.4. The free CaO regions and belite regions derive from the random distribution of raw material particles in the local “volume elements” discussed in a previous section, and the alite regions develop at contact localities between them. The geometry of these regions may differ from location to location in the clinker, but the nature of the contact reaction should be the same.
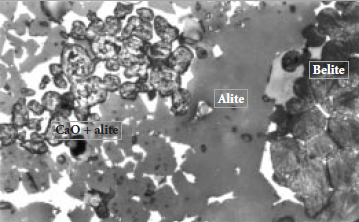
Figure 3.5.4. Free CaO + alite and belite clusters separated by a reaction zone of alite.
In a series of experiments by Christensen and others (1978), and Johansen and Christensen (1979), it was shown that the rate constant for growth of alite reaction zone at the contact localities is pro-portional to the amount of melt content. The rate constant, k, for this experiment as plotted against calculated melt content, α,are shown in Figure 3.5.5. This proportionality shown in the plot is consistent with the mechanism for alite formation in which mass transport (diffusion) through the product layer is the rate-determining factor. This is the mechanism commonly adopted for hetero-geneous high-temperature reactions in condensed systems (Shewmon, 1969; Pask and Aksay, 1975) and was suggested by Nernst in 1904 as a general principle for heterogeneous reactions.
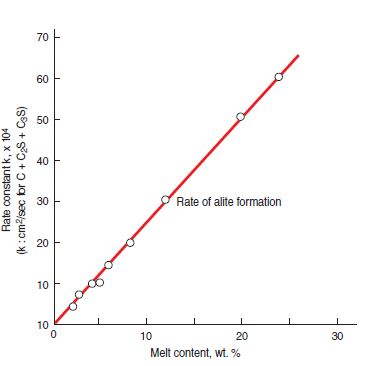
Figure 3.5.5. Rate of alite formation as a function of melt content (After Christensen and others,1978).
If the difference in composition between these “elements” is small, as it would be if cement rock were used as raw material, H is large and the rate of alite formation is high. If the difference in composition were large as it would be if pure limestone and clay with quartz is used, H is small and alite formation is slow. Thus the factor H relates to the two ways of increasing the clinker formation rate mentioned in the introduction, by 1) substantially decreasing particle size of the raw mix, and 2) introducing natural raw materials of unusually high homogeneity and favorable chemical composition.
Taking the rate of growth of the width of the alite region, dx/dt, as a measure for the rate of alite formation in that particular system, differentiation of Equation 7 and substituting for k as in Equation 8, gives:
![]()
The left hand side is proportional to the transport of CaO (or SiO2) through a cross-section in the alite region (Fick’s Law); and the right hand side is written in a way analogous to Ohm’s Law, another transport equation. Ohm’s Law states that the current is proportional to the conductivity and the electrical potential. In Equation 9, dx/dt corresponds to the current, [α·(DL /τ)] corre-sponds to the conductivity, and (∆c·H/x) to the electrical potential. The principal ways of increas-ing the transport rate of electrons or CaO/SiO2,are 1) by increasing the conductivity, and 2) by increasing the potential, which here is synonymous to the driving force, or both.
The role of fluxes is to increase the conductivity,whereas the role of mineralizers is to increase the driving force as will be discussed in the following sections.
Stability of Alite
Effects of CaF2 vs Fe2O3. The fundamental difference between adding 1% Fe2O3 and 1%CaF2 to a raw mix is the possibility of forming alite with CaF2 at temperatures well below the lower stability temperature, To. This is the temperature at which CaO, belite, and alite are in equilibrium and below which alite decomposes to CaO and belite. With CaF2, alite can be formed at as low as 1100°C (Klemm and others, 1978; Christensen, 1979). This is not the case when adding Fe2O3.
At temperatures above the eutectic point, TE,both Fe2O3 and CaF2 accelerate alite formation. However, the increase in reaction rate caused by addition of Fe2O3 is proportional to the increase in melt content, whereas the increase in rate by CaF2 addition is far greater than can be explained by increase in melt content.
Effects of compositions on eutectic points, TE, and melt phases are shown in Figure 3.5.6. A signifi-cant reduction in TE is exemplified by the addition of Fe2O3 and CaF2. Effect of MgO addition as a function of varying silica ratio (SR) and alumina modulus (MA) is also shown for comparison. Significance of alkali and sulfate additions is also shown for some other mix compositions.
Effects of MgO, Al2O3, and Fe2O3. Ludwig and Wolter (1979) showed that the stability temperature of pure C3S decreased from about 1260°C to approximately 1200°C in the presence of 1 mole % MgO, 1 mole % Al2O3, and 1 mole % Fe2O3 in solid solution. The rate of formation of alite was considerably higher as compared to the formation of pure C3S. This indicates that form-ing a solid solution of one or more compounds, which in this case are MgO, Al2O3 and Fe2O3, decreases the lower stability temperature and increases the rate of formation of pure C3S. In a raw mix where alite is already formed, CaF2 does the same but the stability temperature is lowered even more. This effect of lowering the stability temperature and increasing the rate of reaction by form-ing solid solutions has been suggested as a definition for mineralizers as opposed to fluxes which primarily increase the quantity of melt and thereby the rate of reaction.
Christensen (1979) explained that if the mineralizing component enters into solid solution of the product of reactions of the type CaO + C2S → C3S, the rate of reaction will increase, whereas if opposite is the case, i.e., it enters into solid solution of the reactants, the rate will decrease. This is based on thermodynamic considerations of the changes in Gibbs free energy of the reaction. The arguments are as follows (Christensen, 1979). Consider the reaction:
A + B → C
Where,
A, B, and C = Stoichiometric components constituting phases a, b, and c
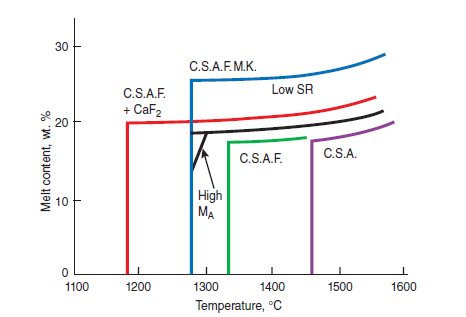
Figure 3.5.6. Effects of composition on melt contents and eutectic points, TE;effects of Fe2O3, CaF2 on the reduction of TE is noteworthy (Adopted from Christensen and Johansen, 1980).
The reaction will proceed with growth of phase c between a and b,controlled by the reaction rates of A and B to inter-diffuse through the product region. It is assumed here that the reaction is diffu-sion controlled as mentioned earlier. The reaction rate is therefore proportional to the diffusion flux, j (e.g. of A, for instance), which can be written as:
j = L ∆µ/∆x
where,
j = Diffusion flux
L = Transport or diffusion coefficient for the specific product region and temperature.
∆µ= Difference in chemical potential of A between the a/c and c/b boundaries
∆x = Distance between these boundaries at the time considered
With increasing amount of fluxing agent, a continuous network of melt phase will develop. Since atomic mobility is much higher in liquid than in solid phase, most of the transport will take place in the melt channels. The value of L therefore will increase drastically. Further increase of flux will result in increase of L, but to a much lesser degree when the melt content is increased from, for instance 20% to 25%, than from 0% to 5%. It can be shown (Christensen, 1979) that ∆µ is identi-cal to – ∆G, the decrease in Gibbs free energy for one mole C formed according to the Equation (10) above and equivalent to the driving force for the reaction.
Now, at the level of 20% melt phase, a mineralizer component is added to the system reacting at a given rate. This component may enter into solid solution in a, b, or c.If it enters into a, the chemi-cal potential of A in phase a will decrease. As an approximation, the potential will decrease by the increment zaRT, where za is the mole fraction of the mineralizer component in phase a; and this will result in a decrease of the driving force and of the rate. However, if it enters into solid solution in phase c, the driving force will increase and so will the rate. This provides, as already stated above, a way of distinguishing between fluxes and mineralizers; mineralizers change the ∆µ (as well as L), whereas fluxes primarily change L; when the mineralizer enters into solid solution of the reactants the rate is decreased, when it enters into solid solution in the product the rate is increased.
For reactions close to an inversion temperature where ∆G (Gibbs free energy) changes sign, such as CaO + C2S → C3S between 1200°C and 1300°C, the value of zaRT is comparable to ∆G. For such reactions the values of ∆G = ∆H – T∆S, and ∆H = To∆S; which upon substitution gives ∆G =
(To –T)∆S. So when a mineralizer causes an increase in the driving force, – ∆G, then To, the lower stability temperature, must decrease. This is in accordance with the observations by Ludwig and Wolter (1979), Klemm and others (1978), and Mukerji (1965). Furthermore CaF2 is known to be more soluble in C3S than in C2S (Lea, 1970; Klemm and others, 1979), and in a system with ~20%melt and addition of 1% – 2% CaF2, the zaRT increment would result in doubling the driving force. This is in accordance with observation that such additions of CaF2 result in doubling the rate of alite formation (Johansen and Christensen, 1979), as is discussed in the following section. In general, increases in ∆µare equivalent to increase of the primary field of the phase under consideration; and the primary field for C3S is indeed wider when the system is doped with CaF2 (Mukerji 1965; Sarkar and others, 1980). This brings us back to the Equation (8) in which ∆c repre-sents the width of the C3S primary field.
FLUORIDE COMPOUNDS
By far the most widely utilized mineralizers in cement manufacturing have been fluoride compounds. The use of fluoride-containing mineralizers has been in practice since late 1800s (Michaelis, 1874; Erdinger, 1882). Investigations were conducted by Kuhl (1924) on the effect of 5%to 10% fluorspar on the clinkering of two cement compositions. The high level of fluorine resulted in a reduction of clinkering temperature and a consequent retardation of cement setting. However, there was little effect on the 28-day strength. Eitel (1938) studied fluorides, whereas Flint (1939) tested fluorides, borates, and phosphates as mineralizers.
Bogue (1947) discussed the use of fluorides as fluxes and mineralizers from the mechanistic and tech-nical standpoints. Fluoride-containing compounds such as CaF2,NaF,BaF2, and MgF2 are all effec-tive fluxes and mineralizers, although CaF2 has found the greatest use. Alkali- and alkaline-earth fluorosilicate salts like Na2SiF6 and MgSiF6 also achieved similar mineralizing effects (Lea, 1971).
Figure 3.5.7 illustrates the effects of CaF2 on widening of the C3S primary field in CaO- SiO2-CaF2 phase diagram. Without CaF2, the primary field of C3S in the CaO-Al2O3-SiO2 phase diagram is restricted to a narrow ‘finger’ as shown in Figure 3.5.8. The widening of the C3S field primarily results from the replacement of Al2O3 by CaF2 (Sarkar and Roy, 1978). This change also reflects a shift in the thermodynamics equilibrium and the rate of alite formation over a range of tempera-ture. Christensen and Johansen (1979) demonstrated that addition of 0.5% to 1% CaF2 signifi-cantly increased the rate of alite formation (Figure 3.5.9). With 1% CaF2 addition, the reaction rate at 1350°C is the same as that as 1480°C without CaF2
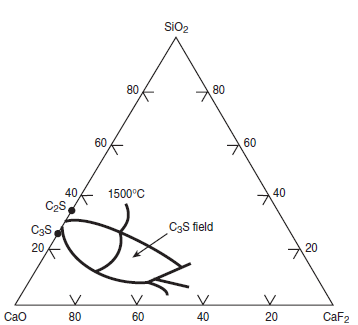
Figure 3.5.7. Relevance of phase diagram CaO-SiO-CaF2 showing (the effect of CaF2 on) the width of C3S field.
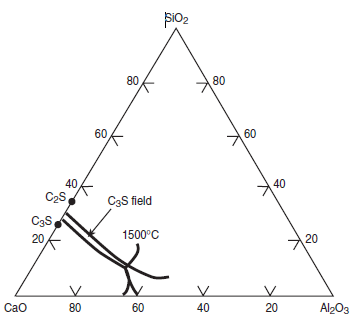
Figure 3.5.8. Phase diagram CaO-SiO-Al2O3 showing the width of C3S field (without CaF2).
Moore (1960) studied natural fluorspar at additions up to 3% by mass of the raw mix as a mineral-izer to boost kiln efficiency in producing low free lime clinkers. Flint (1939), and Ampian and Flint (1973) studied silicofluorides. Since their work demonstrate the effect of mineralizers on C3S formation in a much simpler way, it is discussed below in details.
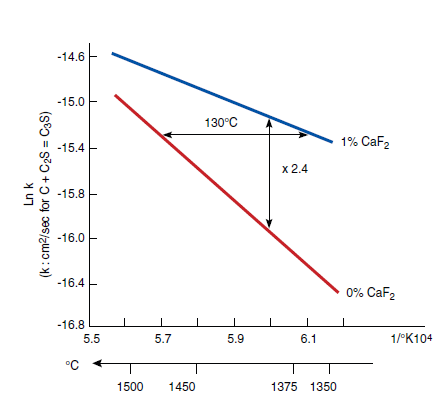
Figure 3.5.9. Effect of CaF2 on rate of Alite formation at various temperatures (After Christensen and Johansen, 1979).
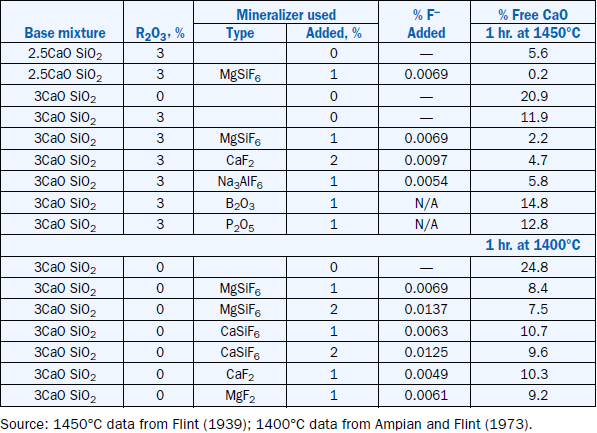
Flint’s 1939 studies investigated the effects of CaF2, MgSiF6,Na3AlF6,B2O3, and P2O5 as mineraliz-ers in the manufacture of portland cement. A synthetic raw mix containing up to 3% R2O3 (equal parts of Al2O3 and Fe2O3 corresponding to 7.90% melt phase at 1450°C according to Equation 6) was burned with 1% or 2% of a given mineralizer for a period of one hour at 1350°C, 1400°C, and 1450°C. The resulting clinker was analyzed for free CaO, and the amounts of C3S, β-C2 S, and
γ-C2S were estimated by microscopy. Table 3.5.4 shows the free CaO after burning for one hour at 1450°C from some of Flint’s experiments with the calculated amount of fluoride, F–, in the miner-alizer. Table 3.5.4 also shows the effect of Al2O3 and Fe2O3 as fluxes by decreasing the free CaO from 20.9% to 11.9% in the base mix 3CaO SiO2 by increasing the R2O3 from 0% to 3%.
Addition of B2O3 and P2O5 had an adverse effect on the CaO consumption, clearly indicating that they are “poison” for the C3S formation. All fluorine-containing compounds had an accelerating effect on CaO consumption and C3S formation (Flint, 1939). MgSiF6 was found to be the most effective mineralizer and CaF2 the least by heating a 3CaO SiO2 base mixture with 3% R2O3 content at 1450°C. Comparing the contribution of F from the mineralizers (Table 3.5.4) to the mixtures, and assuming it enters into solid solution in alite, it would be expected that CaF2 would have the largest effect followed by MgSiF6 and Na3AlF6.However the magnesium and aluminum from the silicofluoride and cryolite respectively would have increased the amount of liquid phase and thereby increased the rate of reaction.
A continuation of this work was reported by Ampian and Flint (1973) in which stoichiometric mixtures of C3S, C2S, C3A, and C4AF were burned with different fluoride containing mineralizers. Results of burning C3S at 1400°C are also shown in Table 3.5.4. Again the mineralizers with magnesium appear as being the most efficient.
Table 3.5.4. Effects of Mineralizers on C3S Formation
One of the consequences of using highly purified reactants and the presence of a low liquid content was the difficulty of completely stabilizing the β-C2S polymorph from forming γ-C2S. β-C2S was stabilized in the mixtures with B2O3 and P2O5 (Flint, 1939). However, Moore (1960) found that when 1% of CaF2 was used on a commercial raw feed rather than a synthetic mix, γ-C2S was not found under any of the test conditions. Klemm and Skalny (1976) also noted that alkali fluorosilicates were as good as, or superior to, CaF2 as mineralizing agents in commercial cement production.
Klemm and Skalny (1976) also made references to a number of researchers who worked on various fluorine-containing compounds as mineralizers. Smaller (0.5% to 1%) amounts of CaF2 as well as NaF and MgF2 were studied by Nagai and Takahara (1936). When added to raw mixes heated at 1200°C to 1350°C for one hour, NaF was found to be the most effective mineralizer, followed by CaF2 and MgF2.According to Longuet and Courtault (1964) liquid formation occurs at 800°C when CaF2 is added to a raw mix. Others have studied the clinkering reactions of belite slime with limestone, and found that a CaF2 flux allowed C3S to form as low as 1200°C. In different studies, both natural and artificial CaF2 were used in amounts ranging from 0.5% to 2% by weight of raw mix to produce a low free lime portland cement clinker. Another study comparing the mineralizing activities of NaF, BaF2, and MgF2 concluded that MgF2 was the best mineralizer. Klemm and others (1979) showed the presence of MgO increased the fluorine content in calcium silicates, which might be the reason for the better observed effects of magnesium silicofluorides.
OTHER ADDITIVES
Although fluorine-containing compounds are proven to be most effective fluxes and mineralizers in the clinkering reactions, a number of studies have been conducted on other possible fluxes as reviewed by Klemm and Skalny (1976), Viswanathan and Ghosh (1983), and Bhatty (1996). Bucchi (1980) has reviewed the effect of minor components as potential fluxing/mineralizing agents; the findings are summarized in the following sections with emphasis on free CaO consumption and alite formation.
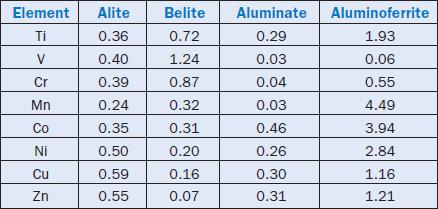
Hornain (1971) investigated the distribution of oxides of transition elements in synthetic clinkers. The elements included titanium, vanadium, chromium, manganese, cobalt, nickel, cupper, and zinc. Mixtures with Bogue calculated values of C3S = 76.3%, C2S = 9.1%, C3A = 5.3% and C4AF = 8.0% were burned at 1500°C for three hours after individual addition of oxides of the components mentioned in amounts of 0.5% oxide. The resulting free CaO was from 0.35% to 0.52%. The clink-ers were analyzed by microprobe and the distribution of the additives between the clinker minerals was determined. Table 3.5.5 shows the results.
Using the distribution between alite and belite shown in the Table 3.5.5, and applying the suggested criteria for characterization of fluxes and mineralizers Ni,Cu, and Zn would be expected to increase the rate for alite forma-tion, Co would almost be without effect and the rest would be inhibitors. Consequently, they would all be expected to contribute to liquid phase formation considering their distribution in the aluminate and alumi-noferrite phases and then act as fluxes.
Table 3.5.5. Distribution of Transition Group Elements in Clinker Minerals, wt. % (Source: Hornain, 1971)
Odler and Abdul-Maula (1980) reported results from a series of burnability experiments and combining these results with Hornain’s (1971) data from Table 3.5.5, Christensen and Johansen (1980) presented a plot in Figure 3.5.8 that demonstrates the accelerating effect on free CaO consumption by transition elements that primarily enter alite in solid solution. The abscissa in Figure 3.5.10 is the solute distribution index, SDI, which is determined as the mole fraction solute in alite minus the mole fraction in co-existing belite (SDI > 0 for element primarily in C3S). The ordinate is the reaction rate index, RRI, determined as free CaO (wt. %) in doped sample after 1 hour at 1300°C minus free CaO (wt. %) in un-doped sample similarly treated (RRI < 0 when burnability is improved). Values for SDI were calculated based on work by Hornain (1971) for distribution of elements between alite and belite. The RRI values were calculated based on work by Odler and Maula (1980). The plot shows that the burnability is improved for raw mixes doped with elements that primarily goes into solid solution of alite.
Imlach (1975) investigated the use of Cr2O3.For many reasons chromium oxide would not be added in commercially manufactured clinker nowadays but the case has scientific interest. Two raw mixes, A and B, made from commercial components with lime saturation factor around 100% were mixed with Cr2O3 from 0.0% to 1.32%, and burned for 30 minutes at 1450°C. The raw mix A was a hard to burn mix, and the resulting free CaO varied from 2.9% to 1.8 % with increasing addition of Cr2O3.Raw mix B was easy to burn and the free CaO after burning at 1450°C for one hour increased from 0.45% to of Cr2O3.Raw mix B was easy to burn and the free CaO after burning at 1450°C for one hour increased from 0.45% to 0.55% with increasing content of Cr2O3.These results can be interpreted as follows. The
fluxing effect is predominant for the burning of mix A. Mix B is so easy to burn that no effect of the extra flux could be registered, whereas the poisoning effect of Cr2O3 Ti caused by primarily entering into belite could be seen.
-4 Viswanathan and Ghosh (1983) made references to work showing that up to 2%Cr2O3 enters into solid solu-tion in alite (contrary to the results of Hornain 1971), and above this limit Cr2O3 caused decomposition of C3S into C2S and CaO. It is also mentioned that β-C2S is stabilized by Cr2O3.
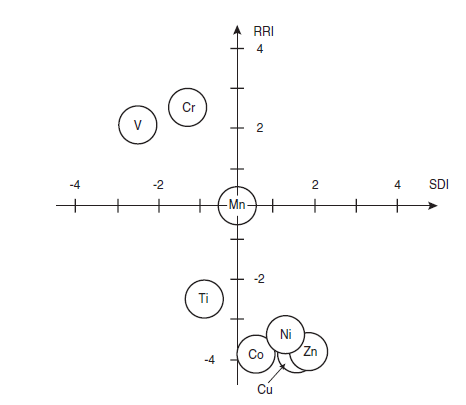
Figure 3.5.10. The relative burnability of raw mixes (RRI) versus the solute distribution (SDI) in alite and belite of certain elements (Adopted from Christensen and Johansen, 1980).
ZnO and ZnS act as fluxes and mineralizers by lowering the clinkerization temperature and accel-erating the lime absorption. This is also what would be expected from the relative distribution of Zn between alite and belite. Titanium added as TiO2 is reported to reduce the temperature of melt formation and thereby act as a flux (Viswanathan and Ghosh, 1983). However, from Table 3.5.5 it would be expected to act as an inhibitor and decrease the rate of alite formation.
The effectiveness of gypsum and sulfate tailings on clinker mineralization has been studied by several researchers as reported by Klemm and Skalny (1976) and Viswanathan and Ghosh (1983). Their addition are reported to have decreased the temperature of melt formation; it is also suggested that gypsum acts as a mineralizer. On the other hand, laboratory tests have also shown that SO3 added as CaSO4 decreased the rate of alite formation. Similar effects were observed when alkalies were added as oxides. However, when alkali sulfates were used, the rate of alite formation was higher than in the cases with either SO3 or alkali oxides (Christensen and Johansen, 1980). In cases where alkalis are not fully combined with SO3 to sulfates, this could be the explanation of the burnability positive effect of adding gypsum to the kiln feed observed in practice. Lawrence (1997) noted that the minor components P2O5,Na2O, K2O, and SO3 primarily enter into solid solution with the belite phase and as a result the rate of CaO combination to alite is slowed down.
MINERALIZER COMBINATIONS
Tewari and Mehta (1972) evaluated the mineralizing effect of fluorogypsum containing about 4% CaF2 in a commercial cement raw mix. At a temperature of 1300°C, clinker samples containing 2% to 3% SO3 from the fluorogypsum additions appeared to be adequately burned and contained less than 1% free CaO. Furthermore, it was found that, because of the SO3 content in the clinker, no additional gypsum was required to regulate the setting and hardening characteristics of the ground clinker. Similarly, Klemm and Skalny (1976) noted that mixtures of gypsum and fluorspar exhibited good mineralizing properties. The practical applications of fluorspar and SO3 is further discussed in the following section.
Klemm and Skalny (1976) referred to work by Simanovskaya and Vodzinskaya (1955) who found that mixtures of P2O5 and Cr2O3 stabilized C3S against thermal decomposition, and accelerated the assimilation of free CaO during the clinkering process. Combinations such as CaSO4 and MgCO3,TiO2 and CaF2,FeSO4 and ZnSO4, and FeSO4 and A12(SO4)3 were also investigated. All showed promising mineralizing affects.
PRACTICAL APPLICATIONS
A survey by Bhatty (1996) on the use of fluxes and mineralizers in North American cement plants and cement plants outside North America, showed some differences between the two groups.
Based on the responses to the survey, the cement plants outside North America seem to use fluxes and mineralizers with better results than the North American plants. The fluxes and mineralizers referred to here were:
1) Fluoride-based materials, primarily fluorspar
2) Non-fluoride materials based on industrial by-products – these contained iron oxide and alumina, and were used as corrective materials to reduce silica ratio (SR) of the raw mix which is equivalent to increasing of the clinker melt and hence improved the burnability.
Bhatty (1996) does not consider iron oxide and alumina containing materials like mill-scale, iron ore, spent aluminum catalyst, blast furnace slag, etc., as belonging to the group of mineralizers because their effect is mainly to increase the content of this melt phase by reducing the silica ratio (SR). However, according to the definition used in this context, such materials are accommodated in the group of fluxes. The findings in the survey by Bhatty (1996) are summarized in the following sections.
North American Cement Plants
Of the North American cement plants at time of the survey only one plant used fluorspar. This was at a level of 0.5 % CaF2 in combination with potassium sulfate, an interesting combination similar to what is used in production of the so-called mineralized clinker (Borgholm and others; 1995 Borgholm 1996), to be described later. The plant operated long dry process kilns and the effect of the mineralizer combination resulted in increased C3S level, reduction of the kiln dust production, overall improved kiln operation, and energy saving of 110 kJ/kg clinker. Other cement plants in the survey used spent aluminum catalyst, iron ore, blast furnace slag, fly ash/mill-scale combination and sewage sludge ash. Most plants experienced improvement in burnability, as would be expected.
One of the plants reported the use of an aluminum catalyst at 0.5% by weight of raw feed in a short kiln with alkali by-pass producing Type I/II clinker. Significant improvements in the burn-ability of the raw feed and in the clinker quality were observed. Another plant used iron ore in a short kiln, and observed improved clinker burnability but no product or energy benefits were reported.
A third plant used blast furnace slag at 4% to 12% levels. The slag contained about 20% Fe2O3 by mass. The plant reported a variety of operational, product, and energy benefits. The use of blast furnace slag improved the burnability, increased C3S, reduced alkalies, and improved grindability (i.e., the clinker became softer). There were also significant energy savings. However, the slag resulted in ring formations in the kiln.
A number of plants were the past users of fluxes and mineralizers, and interestingly enough a majority of these plants had used fluorspar. Common concerns to most of these plants were severe ring formation and/or preheater build-ups which were difficult and costly to remove.
Two plants, each with dry process kilns, used CaF2 at approximately 0.5% by weight of the raw feed. Whereas one plant reported improvement in clinker quality in terms of better compressive strength, the other experienced abnormal setting of cement. They both reported savings in energy, but experienced significant preheater blockage in the lower cyclone stages. Another plant with preheater kiln producing Type I/II clinker, used 0.4% CaF2 by weight of clinker. The plant reported improvement in clinker quality in terms of compressive strength. The plant also reported savings in energy, but experienced significant blockage in the bottom stage of preheaters.
A wet process plant used 0.4% to 0.7% CaF2 to stabilize kiln performance producing Type II clinker. Although there were no kiln build-ups, the CaF2 addition did not benefit the plant with regard to the operation, energy, or product quality. Another plant operating a four-stage preheater kiln used fluorspar to reduce the clinkering temperature in order to overcome excessive build-up in the riser duct. This severely affected the strength performance of clinker.
Two plants, one operating a long wet kiln, and the other with a four-stage preheater kiln and alkali by-pass, also reported using fluorspar. The plants noted a balling effect in the kiln feed. Also noted were the kiln-exit build up and preheater blockage. No reduction in the clinkering temperature or any associated energy benefits were reported.
A plant producing Type III clinker used a combination of fluorspar, gypsum, and salt cake at 1%, 4%, and 0.6% levels by weight of raw feed respectively. The plant reported improvement in clinker in terms of increased C3S content and decreased alkalies. It also recorded energy savings because of reduced clinkering temperature. However, the overriding disadvantage was blockage of the pre-heater at the third stage. Another plant producing Type I clinker used 0.1% CaF2 in a long dry process kiln and reported excessive ring formation that was extremely costly to remove. No prod-uct, operational, or energy benefits were realized.
Another plant used 1% CaSO4 by weight of raw feed. It adversely affected the clinker quality by reducing the C3S level, and decreasing the grindability. The kiln lining was affected because of excessive ring formation. There was no energy saving; moreover the SOx level in the emissions increased. A wet process plant tested zinc sludge as a flux/mineralizer. The addition was done in the mid-kiln section. The clinker quality improved. Compressive strengths increased at all ages. Both the normal setting times and the false setting characteristics of cement improved. Zinc was substituted in the C4AF phase. Implications on energy saving were not reported.
A summary of select data on the use of fluxes and mineralizers in North American cement plants is shown in Table 3.5.6.
Table 3.5.6. Summary of Data on Fluxes and Mineralizers Used by North American Cement Plants (Bhatty, 1996)
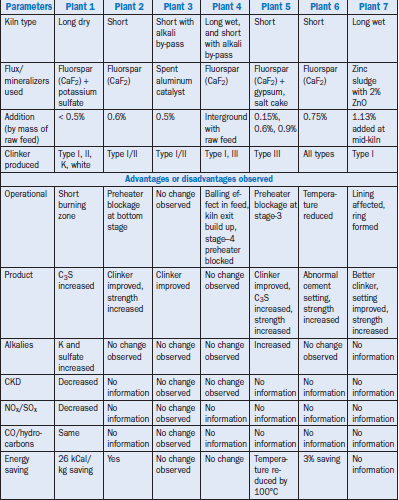
Cement Plants Outside North America
At the time of survey several responding cement plants outside the North American continent used fluxes and mineralizers. A majority of these plants used fluorspar-based materials.
One plant operated a precalciner kiln using 0.3% CaF2 by weight of raw feed. The addition resulted in several operational, product, and energy improvements. Overall production and grind-ability of clinker improved. However, blockage in the second stage of the preheater was also recorded. The setting time of the cement increased, which was countered by sulfate addition.
A number of plants with preheater, precalciner, and alkali by-pass, reported using 0.2% CaF2 by weight of raw feed with iron oxide in various modifications. The material is primarily interground with the raw feed, although introduction through the burner pipe has also been reported. The use resulted in improved operation, production, and energy consumptions. Overall production in-creased by 5% to 10%, and the quality of clinker improved with increase in C3S level (though at the expense of grindability). There were significant energy savings (2% to 4%). The levels of NOx and CO emissions also decreased.
Three plants with two suspension and one grate preheater kilns used 0.4% to 0.5% CaF2 with improved kiln operation, cement quality, and energy savings as a result. No ring formation or preheater blockages were encountered. Burning temperature was reduced by 50°C and there has been an energy savings of up to 90 kJ/kg of clinker. Production increased by up to 10%. C3S content in the clinker increased and strength improved. NOx emissions were reduced. Clinker grindability was somewhat adversely affected.
Two separate groups of cement plants in Europe reported the use of fluoride-based fluxes and mineralizers with significant operational, and energy benefits; they apparently had no production or product problems. Three other cement companies reported the use of iron-containing materials and they all experienced improved burnability; two of the plants also reported energy savings. Problems with ring formation or preheater build-ups were also encountered. One company oper-ating long dry kilns, preheater kilns, and Lepol kilns reported testing fluxes/mineralizer in the past. They abandoned the tests after concluding that their use was unjustified.
Erhard (1994) reported an increase in kiln production from 5% to 10% and reduction in fuel consumption in the order of 2.3% – 4.5% by using CaF2 contents in kiln feed of 0.25% – 0.30%.
A summary of select data on the use of fluxes and mineralizers in cement plants outside North America is given in Table 3.5.7.
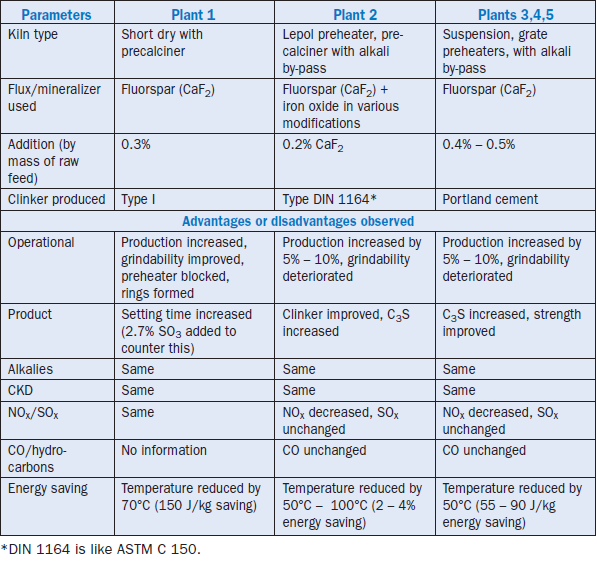
Table 3.5.7. Summary on Fluxes and Mineralizers Used by Cement Plants Outside North America (Bhatty, 1996)
MINERALIZED CLINKERS
Mineralized clinker, based on development at the former Blue Circle Industries PLC, is produced in Denmark (Borgholm and others, 1995; Borgholm, 1996), and other countries. The mineralizer used is a well-controlled mixture of CaF2 and gypsum. The development work as well as the result-ing commercially produced clinker and cement is already well described by Moir (1982), Borgholm and others (1995), and Borgholm (1996). Since it is an example of laboratory work resulting in actual production, it is worthwhile looking at in some detail.
The important parameter is the combination of fluoride and gypsum. Laboratory test showed that adding the mineralizer pair reduced free lime content from 5% for the undoped mix burned at 1450°C to 2% after burning at 1370°C. Higher levels of mineralizer addition could lower the temperature further, but considerations on clinker quality and practicability of kiln operation set limits to how high the levels of fluoride and SO3 can be allowed.
The raw mixes used typically had high lime saturation factor and silica ratio. Addition of K2SO4 was made to control the K2O:SO3 ratio. The development of phases at temperatures higher than 800°C was monitored. In this regard Moir (1982) emphased:
“It is important to note that these are unlikely to be equilibrium phase assemblages, as the samples were not held at the final burning temperature. It should also be remembered that many of these phases will not have been present at the temperature indicated but formed on cooling.”
The C3S was first detected at 1270°C; the fluoro-aluminate phase C11A7 CaF2 existed only between about 1170°C and 1280°C. The raw mixes were made with calcium fluoride along with sulfate addi-tion. For raw mix with LSF = 102.4%, SR = 5.8, SO3 = 3.6%, and F2 = 0.25%, the free lime in clinker reached a maximum of 38% at 1050°C and then decreased to 1.9% at 1450°C. The alite was present in the rhombohedral form.
The effect of fluoride on the clinker reduced the hydraulic activity at early ages. The one-day strengths, however, reached maximum at addition levels of 0.5% CaF2.Moir (1982) noted that the optimum addition levels of CaF2 were higher at later ages, “This is probably analogous to the phenomenon of increased late strengths commonly found when the rate of hydration at early ages is reduced by low temperature curing conditions or by the use of retarders. Further evidence in early hydraulic activity accompanying the increase in clinker fluorine levels comes from the progressive increase in setting times.”
Following this work, production of mineralized clinker was commenced at a Danish cement plant in a 5500 tpd semi-dry, two stage preheater calciner kiln. Because of the decreased burning zone temperature and relatively high excess air equivalent to high oxygen partial pressure, the volatility of alkalies and sulfate could be controlled. As a consequence, inexpensive fuels with high sulfur content could be tolerated. The plant reported a marginal fuel savings of about 3%, and a 50%reduction in NOx emissions compared to when producing non-mineralized clinker (Borgholm and others, 1995). Trouble free production of the mineralized clinker to avoid cyclone blocking and dust circulation between cooler and kiln primarily dependent on the control of the mineralizer mix relative to the alkalies of clinker. If the clinker alkali content is higher than 0.8 Na2Oeq and the SO3 content is correct, the setting of mortars and concrete is effectively controlled by the calcium langbeinite formed in the clinker, and the need for gypsum addition to the cement is reduced or eliminated. This makes the temperature control of the finish mill less critical (Borgholm and others 1995; Borgholm, 1996). It is further reported that the specific energy for grinding the clinker is low, and the particle size distribution is steep, which results in rapid strength development.
Photomicrograph of polished section and SEM image of select mineralized clinkers are shown in Figure 3.5.11 and Figure 3.5.12 respectively. Typical rhombohedral alite and round belite crystals (with lamellae) are shown distributed in the clinkers; anhydrite, sulfate melt, and calcium langbei-nite phases in the clinker are also shown.
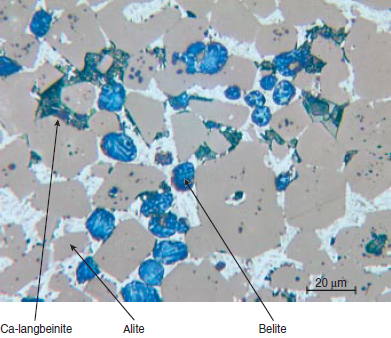
Figure 3.5.11 Polished section of a mineralized clinker (Photo courtesy Duncan Herfort, Ålborg Portland).
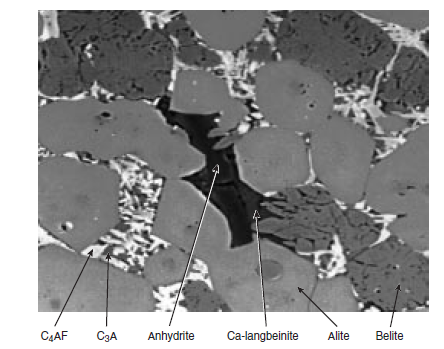
Figure 3.5.12 SEM image of polished section of the mineralized clinker containing 1.5% anhydrite(3% SO3) (Photo courtesy Duncan Herfort, Ålborg Portland).
POSSIBLE SCENARIOS
The most common concern reported in the North American industry, when using the fluoride-based fluxes/mineralizers, was the blockage of preheater cyclones caused by fines and volatiles. It is possible that while conducting the trials with fluorides, the optimal reduction and control of the kiln temperature may not have been achieved. Thus, the heat that should have been conserved by lowering the kiln temperature may have resulted in an unfavorable thermal profile in the kiln caus-ing overheating of the load and the generation of additional volatiles and fines. Such overburning could also produce clinker that is difficult to grind. The temperature of the kiln could possibly be reduced to compensate for the fluxing and mineralizing effect. This would conserve thermal energy and reduce particulates to avoid plugging of preheaters, while producing properly burned clinker.
Other possible concerns might be related to the quality of cement produced from mineralized clinker; these include adverse hydraulic activity and early compressive strength. If the mechanism of mineralizers is as discussed above, these effects might be expected. It is generally accepted that fluorine delays the setting time, however, without necessarily affecting the later strength develop-ment. As mentioned above by Moir (1982), the effect is similar to the use of retarders or curing at low temperatures, but the later-age strengths are generally increased. Accordingly, clinkers pro-duced by Erhard (1994) using 0.25% – 0.30% CaF2 in the kiln feed, gave cement higher strength than clinker burned without fluoride. However, he cautions that the control of the mineralizer amount is critical, too high a content will result in significant increase of setting time and decrease of early strengths, in particular the 1-day strengths.
A CRITICAL COMMENT
Although the pyroprocessing of cement raw feed into clinker is energy intensive, the energy consumption for a modern cement plant with multiple preheater stages and calciner configura-tions can be as low as 2.94 GJ/t (700 kcal/kg). It should also be kept in mind that the energy involved in the calcination of a typical cement raw feed is 1.75 GJ/t (420 kcal/kg). Thus, the poten-tial for energy savings by decreasing clinkering temperatures and increase reaction rates are rela-tively small for the modern kiln systems. Therefore, the use of fluxes and mineralizers in conserving the thermal energy by means of reducing the clinkering temperature might not be that significant. The possible advantages of using fluxes and mineralizers can rather be in terms of increased production which in turn will decrease specific heat losses, reduce emissions, and extend specific brick life, thereby improving the overall energy and cost efficiency (Lawrence 1998).
According to Gardeik (1981), less than 5% energy saving can be expected with a decrease of burn-ing temperature by 200°C, and that the incentive to use mineralizers may come from other consid-erations such as:
• Reduced processing cost by preparing coarse raw mix
• Extended refractory life as result of reduced temperature and retention time, or
• Improvement in cement quality.
CONCLUSIONS
Despite promising results in cement manufacture, industry has yet to implement a meaningful use of fluxes and mineralizers in practice. The fluxes primarily increase the amount of melt phase and improve the burnability of a kiln feed. The agglomeration and nodulization may also be improved. However, if a flux forms too high an amount of melt phase, clinker balls and ring formation may cause problems. Mineralizers cause an increase in the rate of alite formation and stabilize it. From a practical standpoint this is equivalent to an improvement in the kiln feed burnability, similarly to the effects of fluxes.
Furthermore, the fact that the melt phase forms over a narrow temperature range puts practical limits to how much the burning zone temperature can be lowered and how close to the critical temperature a kiln can be safely operated.
There are two ways of taking advantage of the improved burnability. One is to decrease the temperature, and the other is to maintain the temperature and shorten the retention time. This is equivalent to increasing the throughput of a kiln and thereby increasing production. So, in practice the benefit may not be so much of decreasing the burning zone temperature as it would be of increasing the production. Shortening the retention time by increasing the rate of material flow, which is equivalent to increasing production, will also result in the reduction of the specific fuel consumption because of the reduced heat losses from the cooler, kiln shell, and preheater. This, in turn, will reduce heat losses from the exhaust gas and also reduce stack emissions.
REFERENCES
Ampian, S. G., and Flint E. P., “Effect of silicofluorides on the Formation of Calcium Silicate, Alu-minates and Aluminoferrite,” The American Ceramic Society Bulletin, Vol. 52, pages 604-609, 1973.
Alsop, P. A.; Chen, H.; Chin-Fatt, A. L.; Jackura, A. J.; McCabe, M. I.; and Tseng, H. H., Cement Plant Operations Handbook, Tradeship Publications, Ltd., Surrey, U.K., 2001.
Bhatty, J. I., Use of Fluxes and Mineralizers in the Cement Industry: A Survey, Portland Cement Association R&D Serial No. 2045, 27 pages, 1996.
Bogue, R. H., The Chemistry of Portland Cement, Rheinhold Publishing Corporation, New York, U.S.A., 1947.
Borgholm, H. E.; Hertford, D.; and Rasmussen, S., “A New Blended Cement Based on Mineralized Clinker,” World Cement Research and Development, pages 27-33, August 1995.
Borgholm H. E., “Better, But How?,” International Cement Review, pages 66-68, June 1996.
Butt, Y. M.; Timashev V. V.; and Ozokin, A. P., “The Mechanism of Clinker Formation Process and Ways of Modification of Clinker Structure,” Principal Paper, 6th
International Congress on Chemistry of Cement, Moscow, Section I, 75 pages, 1974.
Christensen, N. H.; Jepsen, O. L.; and Johansen, V.,“Rate of Alite-Formation in Clinker Sandwiches,” Cement and Concrete Research, Vol. 8, No. 6, pages 693-702, 1978.
Christensen, N. H., and Johansen, V.,“Mineralizers and Fluxes in Clinker Formation. II. Kinetic Effects on Alite Formation,” 7th International Congress on Chemistry of Cement, Paris, Vol. II, Theme I, pages 1-5, 1980a.
Christensen, N. H., and Johansen, V., “A Model for the Clinker Reaction,” Poster Presentation, 7th International Congress on Chemistry of Cement, Paris, 1980b.
Christensen, N. H., “Modelling the Clinker Reaction,” World Cement Technology, Vol. 12, No. 5, pages 238-247, June 1981.
Christensen, N. H., and Johansen, V., “Role of Liquid Phase and Mineralizers,” Cement Production and Use, Ed. Skalny, Engineering Foundation , New York, Vol. 8, No. 6, pages 55-59, 1979.
Chromy, S., “Mechanism of White Clinker Formation,” Supplementary Paper III-8, 6th International Congress on the Chemistry of Cement, Moscow, 9 pages, September 1974.
Egeloev, A. H., and Petersen, I. F., “Mechanical Properties of Clinker at High Temperatures,” Zement-Kalk-Gips, Vol. 34, pages 591-594, 1981.
Erhard, H. S., “Fluxes and Mineralizers in the Clinkering Process – Operational Results with Flourides,” Portland Cement Association General Technical Meeting, Seattle, U.S.A., September 18-21, 1994.
Flint, E. P., “Mineralizers in Cement,” Rock Products, Vol. 42, pages 40-52, 1939.
Fundal, E., “The Burnability of Cement Raw Mixes,” World Cement Technology, Vol. 42, pages 40-52, July/August 1979.
Gardeik, H. O., “Effect of the Clinkering Temperature on the Specific Energy Consumption in Cement Clinker Burning,” Zement-Kalk-Gips, Vol. 4, pages 169-174, 1981.
Grachian, A. N.; Zubekhin, A. P.; and Leonov, V. M., “Dependence of the Liquid Phase Viscosity of a Cement Clinker on the Characteristics of Cations and Anions of Mineralizers,” Zh. Prikl. Khim, Leningrad, Vol. 44, page 189, 1971.
Gutt, W., and Osborrne, G. J., “The Calcium silicofluorides of Tentative Composition
3CaO.SiO2.3CaF2,” Transactions of British Society, Vol. 67, No. 4, pages 125-133, 1968.
Johansen, V., “Application of Equilibrium Phase Diagrams to Industrial Clinker Formation.” Zement-Kalk-Gips, Vol. 32, No. 4, pages 176-181, 1979.
Johansen, V., and Christensen, N. H., “Rate of Formation of C3S with Addition of CaF2,” Cement and Concrete Research, Vol. 9, No. 1, pages 1-6, 1979.
Klemm, W. A.; Jawed, I.; and Holub K. J., “Effects of Calcium Flouride Mineralization on Silicates and Melt Formation in Portland Cement Clinker,” Cement and Concrete Research, Vol. 9, pages 489-496, 1979.
Klemm, W. A., and Skalny, J., “Mineralizers and Fluxes in Clinkering Process,” Cement Research Progress, American Ceramic Society, Columbus, Ohio, pages 259-280, 1976.
Kuehl, H., “Addition of fluorspar to Cement-Mix,” Zement, Vol. 13, No. 2, pages 9-10, 1924.
Kuehl, H., Zement-Chemie, Band II, 1952.
Lawrence, C. D., The Production of Low-Energy Cement, Lea’s Chemistry of Cement and Concrete, Ed. P. C. Hewlett, John Wiley & Sons, New York, pages 421-470, 1998.
Lea, F. M., and Parker, T. W, Building Research Technical Paper No. 16, His Majesty’s Stationary Office, London 1935, also in Lea, F. M. The Chemistry of Cement and Concrete 3rd Ed. E. Arnold, Glasgow, 1970.
Ludwig, U., and Ruckensteiner, G., “Einflüsse auf die Brennbarkeit von Zementrohmehlen,” Westdeutscher Verlag, Opladen 1973.
Ludwig, U., and Wolter, A., “The Formation and Stability of Tricalciumsilicate and Alites,” Zement-Kalk-Gips, Vol. 32, No. 9, pages 455-459, September 1979.
Longuet, P., and Courtault, B., “Suitability of Cement-Plant Raw Mixes for Clinker Formation,” Rev. Mater. Construct, Trav., No. 585, pages 174-187, 1964.
Michaelis, W., “Notizblatt der Vereins fuer Fabrikation von Ziegeln, Tohnwaren und Zement,
Nr 3., 1875.
Mishulovich, A., Halides as Catalysts for Calcination, Portland Cement Association, R&D Serial No.1991, 1994.
Moir, C. K., “Mineralized High Alite Clinker,” World Cement, Vol. 13, No. 10, pages 374-382, December 1982.
Moore, R. E., “Fluorspar Boosts Kiln Efficiency,” Rock Products, Vol. 63, No. 12, pages 108-112, 1960.
Mukerji, J., “ Phase Equilibrium Diagram CaO- CaF2-2Ca.SiO2,” Jr. Amer. Ceram. Soc., Vol. 48, pages 210-213, 1965.
Nagai, S., and Takahara, M., “The Effect of Various Flourides on the Thermal Combination of Portland Cement Raw Mixtures,” Jr. Soc. Chem. Ind. Japan, Vol. 39, pages 183-184, 1936.
Nernst, W. Z., Phys. Chemie, Vol. 47, pages 52-55, 1904.
Okorokov, S. D.; Golynko-vol’fson, S. L.; Sheveleva, B. I.; and Yarkina, T. N., “Comparison of Salt Groups as Potential Mineralizers in Firing Portland Cement Clinker,” Tsement, Vol. 3, pages 5-11, 1957.
Osbaeck, B., Private Communication, 2000.
Pask, J. A., and Aksay, I. A., Mass Transport Phenomena in Ceramics, Eds. A. R. Cooper and A. H. Heuer, Plenum Press, New York, 1975.
Petersen, I. F., “Isothermal Sintering of Portland Cement Raw Mixes – Part 1,” World Cement, Vol. 14, No. 5, pages 188-196, June 1983a.
Petersen, I. F., “Isothermal Sintering of Portland Cement Raw Mixes – Part 2,” World Cement, Vol. 14,No. 6, pages 220-228, July/August 1983b.
Pliego-Cuervo, Y. B., and Glasser, F. P., “The Role of Sulfates in Cement Clinkering Reactions: Phase Formation and Melting in the System CaO-Ca2SiO4-Ca2SO4-K2SO4,”
Cement and Concrete Research, Vol. 7, No. 5, page 477, 1977.
Pliego-Cuervo, Y. B., and Glasser, F. P., “The Role of Sulfates in Cement Clinkering Reactions: Phase Formation in the System CaO-Al2O3-Fe2O3-SiO2-Ca2SO4-K2SO4,”
Cement and Concrete Research, Vol. 9, No. 5, page 573, 1979.
Ponomarev, I. F.; Grachian, A. N.; and Zubehkin, A. P.,“Influence of Mineralizing Additives on the Process of Cement Clinker Formation as a Function of the Electroneutralitivity of the Mineralizer Cation and Anion,” Dokl. Akad. Nauk. SSSR, Vol. 166, No. 2, pages 410-412, 1966.
P’yachev, V., and Tikhonenkova, Z. V., “Assimilation of Lime During synthesis of clinker Mineralsand Calcination of Clinker in the Presence of Addition of Cr2O3,B2O3,P2O5,V2O5,” Izv. Vyssh. Ucheb. Zaved. Khim. Khim. Tekhnol., Vol. 9, page 410, 1966.
Shewmon, P. G., Transformation in Metals, McGraw-Hill, New York, 1969.
Shubin, V. I., “Investigation into Consolidation of Portland Cement Clinker,” Supplementary Paper, 6th International Congress on the Chemistry of Cement, Moscow, Section I, Theme 5,18 pages, 1974.
Simanovskaya, R. E., and Vodzinskaya, Z. V., “Influence of Fluorine in Presence of Phosphates on Formation and Crystallization Reactions of Clinker Minerals,” Tsement, Vol. 5, pages 12-14, 1955.
Sorrentino, F. P., Glasser, F. P. (1975), “The System CaO-Fe2O3-Al2O3-SiO2:I.The Pseudoternary Section Ca2Fe2O5-Ca2Al2O5-SiO2,” Trans. Jr. Brit. Ceram. Soc., Vol. 74, No. 7, pages 253-256, 1975.
Sprung, S., and Von Seebach, H. M., “fluorine Balance and fluorine Emission from Cement Kilns,” Zement-Kalk-Gips, Vol. 21, No. 1, pages 1-8, 1968.
Taylor, H.F.W.,Cement Chemistry, 2nd Edition, Thomas Telford, 1997.
Teoreanu, I., “Effect of Flouride Constituents on the Formation of Crystals and on Composition of the Mineralogical Constituents of Clinkers in Portland Cement,” Rev. Mat. Constr. Trav., Publication 666, pages 73-77, 1971.
Teoreanu, I., “The Chemistry of White and Colored Cements,” Principal Paper, 6th International Congress on the Chemistry of Cement, Moscow, Section III, 58 pages, 1974.
Timashev, V. V., “The Kinetics of Clinker Formation. The Structure and Composition of Clinker and its Phases,” Principal Paper, 7th International Congress on the Chemistry of Cement, Paris, Vol. I, Theme I, pages I–3/1-3/20, 1980.
Timashev, V. V., and Albats, B. S., “The Process of Liquid Phase Sintering of Portland Cement Clinker,” 6th International Congress on the Chemistry of Cement, Moscow, Supplementary Paper Section I, Theme 3, 16 pages, 1974.
Tsuboi, T.; Ito, T.; Hokinous, Y.; and Matsuzaki, Y., “The Influence of MgO, SO3 and ZnO on the sintering of Portland Cement Clinker,” Zement-Kalk-Gips, Vol. 25, No. 9, pages 426-431, 1972a.
Tsuboi, T., and Ogawa, T., “Microscopic Studies of Clinker for Evaluating the Sintering Process,” Zement-Kalk-Gips, Vol. 25, No. 6, pages 292-294, 1972b.
Viswanathan, V. N., and Ghosh, S. N., “Mineralizers and Fluxes in Clinkerization,” Advances in Cement Technology, Ed. S. N. Ghosh, Pergamon Press, pages 177-202, 1983.