Contents
Everything you need to know about Minor Elements in Cement Manufacturing
by Javed I. Bhatty*
Minor elements are derived from the raw materials and fuels used in cement manufacture, for example, limestone, clay/shale, and coal. They also come from widely used auxiliary materials such as blast furnace slag, fly ash, iron oxide, bauxite, and spent catalysts. A secondary but important source of minor elements is the industrial wastes that are used for supplementation of the primary fuel. These include petroleum coke, used tires, impregnated sawdust, waste oils, lubricants, sewage sludge, metal cutting fluids, and waste solvents.
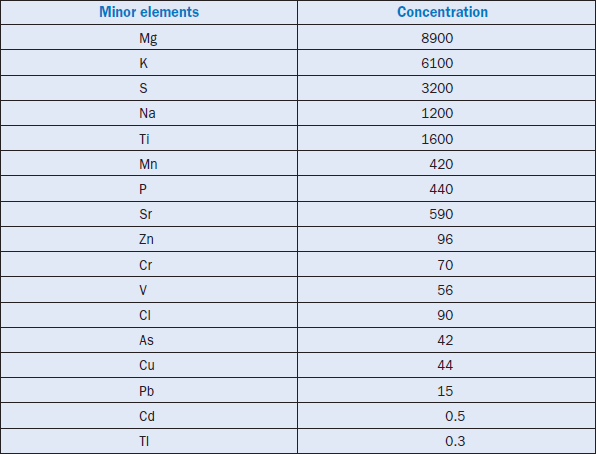
Minor elements found in a typical raw feed for cement manufacturing as quoted by Bucchi (1980) are shown in Table 3.6.1. Similar data for limestone and shale/clay and some auxiliary raw minerals such as blast furnace slag and coal fly ash are shown in Tables 3.6.2 and 3.6.3. Minor elements found in conventional kiln fuel (coal), along with two secondary fuels (used oil and petroleum coke), are shown in Table 3.6.4, and those found in typical clinkers (Moir and Glasser, 1992) are given in Table 3.6.5. A commercial clinker containing chromium is shown in Figure 3.6.1. The effect of chromium on belite and alite formation in the clinker is noteworthy.
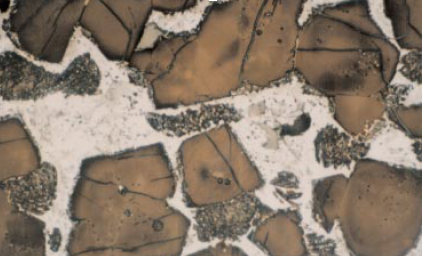
Figure 3.6.1. Effect of chromium on the formation of decomposed alite and dendritic belite in clinker. The alite crystals are, however, enlarged.
Table 3.6.1. Typical Minor Elements Present in a Cement Raw Meal (from Bucchi, 1980)
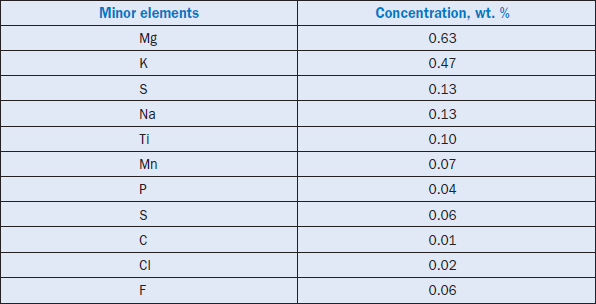
Table 3.6.2. Concentrations (ppm) of Minor Elements in Limestone and Clay/Shale (from Sprung, 1985)
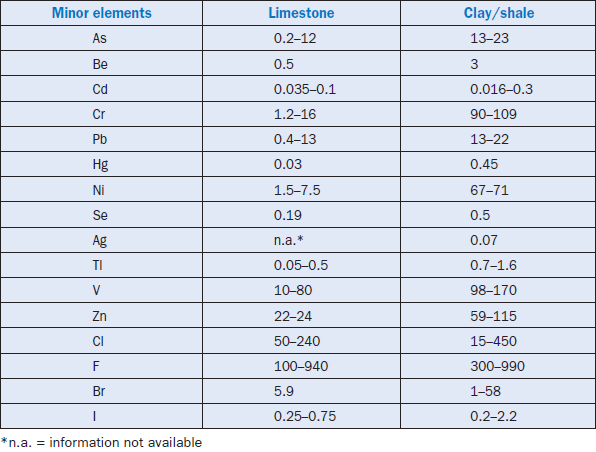
Table 3.6.3. Concentrations (Wt. %) of Minor Elements in Auxiliary Raw Materials (from Moir and Glasser, 1992; Smith and Others, 1979)
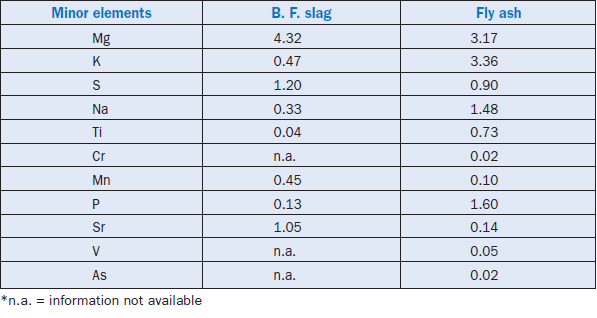
Table 3.6.4. Concentrations (ppm) of Select Minor Elements in Coal, used Oil, and Petroleum Coke (from Sprung, 1985; Weisweiler and Krˇcmar, 1989)
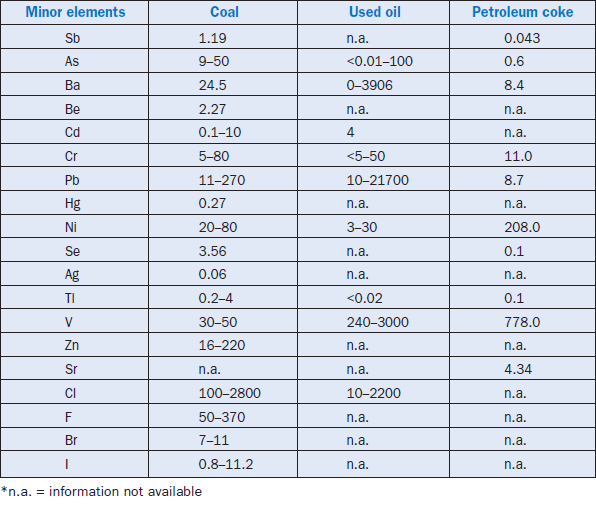
Table 3.6.5. Average Concentrations (ppm) of Minor Elements in Typical Clinkers (from Moir and Glasser, 1992)
Although blast furnace slag can be used up to 30% by weight, the level of use may be restricted by its magnesium oxide (MgO) content, particularly if the MgO level is already high in the raw mate-rials. Bauxite is typically reported to contain 2%-8% titanium oxide (TiO2) and 0.04%-0.4%chromium oxide (Cr2O3). Iron ores frequently contain chromium, arsenic, cadmium, and thal-lium, and thereby may have environmental consequences because of the toxicity characteristics and related emission problems.
The levels of sulfur and chlorine in bituminous coal vary from 0.5%-4% and 0.007%-0.39%respectively. In some coals, sulfur is present up to 6% by mass. Petroleum coke frequently contains up to 6% sulfur or more and 0.1% vanadium oxide (V2O3), and can contribute to increased levels of sulfur and vanadium to clinker when used as a supplement for coal. Scrap tires, used as supple-mentary fuel, have a high zinc content of 1.0%-1.6%. However, if tires replace 10% of the primary fuel, the resulting zinc oxide (ZnO) contents in clinker would increase by only 0.02%.
Additional sources of minor components could be the refractories and the grinding media such as liners and grinding balls. A damaged magnesite-chrome refractory lining can be incorporated into the incoming raw mix and result in a measurable amount of chromium into the clinker. Since this chromium is often in the toxic +6 (hexalent) state, the use of chrome bricks is being phased out in most parts of the world.
DEFINITIONS
Minor Elements
Elements in clinker at concentrations less than 1% by mass are regarded as minor elements (Miller, 1976; Gartner, 1980). They are generally categorized on the basis of the frequency with which they occur in raw materials and the eventual mix feed. Elements occurring at less than 0.02% by mass are classified as trace elements (Blaine and others, 1965); however, Sprung (1988) has termed elements present at less than 100 ppm level as trace elements. In this chapter, however, minor and trace elements have been grouped together and discussed without distinction. Earlier reports by Gartner (1980) and Bhatty (1995) have extensively reviewed the role of minor elements in cement manufacturing and use.
Lesser Elements
Elements appearing in almost every commercial portland cement at levels 0.15%-5% by mass are sodium (Na), potassium (K), sulfur (S), and magnesium (Mg). They are termed as lesser elements. Rompps Chemie-Lexicon (1987) has termed them as secondary elements. Discussion of these elements has also been included in this chapter where appropriate.
Major Elements
In cement analyses, Ca, Si, Al, Fe, and O are expressed as the major elements since their reported concentrations in clinker can be beyond 5% by mass (Rompps Chemie Lexicon, 1987). They typi-cally occur as oxides: CaO, SiO2,Al2O3, and Fe2O3.However, they exist as more complex compounds in cement. The approximate formulae of these compounds, also known as clinker phases, are: tricalcium silicate 3CaO·SiO2 or C3S*; dicalcium silicate 2CaO·SiO2 or C2S; tricalcium aluminate 3CaO·Al2O3 or C3A; and tetracalcium aluminoferrite 4CaO·Al2O3·Fe2O3 or C4AF.
Since this chapter only deals with minor elements, and the role of major elements in cement manufacturing is fairly well documented, only a summary of the major elements, Ca, Si, Al, and Fe, is included here.
Calcium, as CaO or lime, is an essential component of cement, which comes from the decomposi-tion of major raw materials such as limestone, chalk, marl, cement rock, or even dolomite depend-ing upon the geological location of the cement manufacturing plant. Silicon as SiO2 in cement is derived from silica sand, or from clay, shale, or slate, which are also sources of aluminum as Al2O3, and iron as Fe2O3 in the raw material. Fe2O3 is sometimes derived from iron ores, or mill scale, and added separately if the raw mix is deficient in iron. Al2O3 may be added with bauxite or other sources. Auxiliary materials such as fly ash and blast furnace slag are also often added as raw feed substitutes.
A ground mixture of the raw materials containing major components in the desired proportions is converted to clinker by burning in a rotary kiln at about 1450°C, when the constituents become fully oxidized and form stable solid phases C3S, C2S, C3A, and C4AF as mentioned above. Impure C3S is also frequently known as alite, and C2S as belite. After cooling, the clinker is interground with approximately 5% gypsum to about 350 m2/kg Blaine fineness, to obtain portland cement. A typical composition of ASTM Type I cement, the most commonly used cement in general con-struction, is nominally 50% C3S, 25% C2S, 12% C3A, 8% C4AF, and 5% gypsum. There are also other types of cement such as Type II, III, IV, and V cements which vary markedly in composition and are used where special properties are required.
ROLE OF MINOR ELEMENTS
The interaction of minor elements coming from the primary and auxiliary raw materials as well as the conventional and secondary fuels during cement manufacturing is discussed here. The discus-sion in this chapter pertains to the role of both the common and uncommon minor elements that have significance in cement manufacturing.
Aluminum
The role of aluminum (Al) in cement manufacturing has been well understood, however, it is briefly discussed earlier in the section of major elements.
Antimony
Antimony (Sb) occurs as traces in cement raw materials. It has been reported to occur at 0.08 ppm in the raw feed and 0.0429 ppm in petroleum coke (Weisweiler and Krˇcmar, 1989).A considerable portion of antimony gets incorporated in clinker in the form of low volatile calcium antimonates under oxidizing kiln conditions at high temperatures (Weisweiler and Krˇcmar, 1989). The oxides, Sb2O3,natural seranmontite, and valentinite, are not very volatile at kiln temperatures; they sublime at 1550°C. However, some high levels of Sb have been reported in the kiln dust from some cement plants by Lee and von Lehmden (1993); Gartner (1980) argued that the limited nature of this data might not be very reliable.
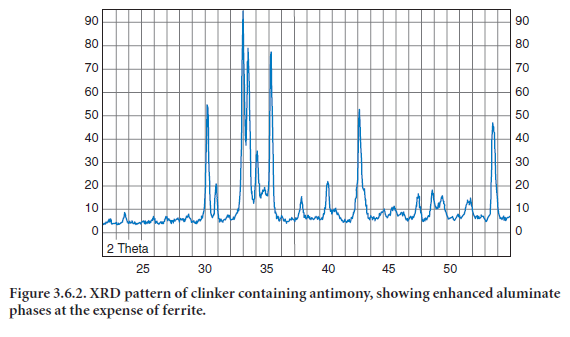
Bhatty (2003) has shown that the addition of antimony reduces the formation of ferrite to the benefit of aluminates, (Figure 3.6.2). Additionally, alite formation is improved, however the size of both alite and belite are reduced, (Figure 3.6.3).

Arsenic
Arsenic (As) bearing minerals arsenolite or claudite As2O3 (or As4O6) occur only in small amounts in coal and used oils, and are unlikely to influence cement manufacturing in any way. Smith and others (1979) have indicated that in coal-fired power plants, both As and Sb tend to concentrate in the fly ash, but their concentration levels are extremely low. It tends to concentrate in the fine fractions of fly ash (Coles and others, 1979). Weisweiler and Krˇcmar (1989) have reported up to 5 ppm of As in raw material and only 0.6 ppm in petroleum coke. Arsenic levels found in various materials are shown in Tables 3.6.2 to 3.6.4.
Although As2O3 is volatile (sublimes at 193°C) and if present as such should be expected to condense on kiln dust particles, Weisweiler and Krˇcmar (1989) observed that a substantial amount of As is incorporated in the clinkers, and only a negligible portion of As ends up in the dust. The cause of As entering into clinker was attributed to excess CaO, oxidizing conditions in the kiln, and high kiln temperature. Under oxidation conditions, As is primarily oxidized to As2O5 and forms a series of low volatile calcium arsenates, among which Ca3(AsO4)2 is more stable at 1300°C. Czamarska (1966) found that 0.15% As5+ significantly decreased the rate of C3S formation at 1450°C.
Moir and Glasser (1992) reported that owing to the toxic and volatile nature of As and its potential release in stack emissions, the cement industry as a whole is maintaining control over the As levels in clinker so that the adverse effects on environment and cement properties are negligible.
Barium
Barium (Ba) occurs in varying amounts in limestones, mostly as barite (BaSO4). It can also occur in clayey sediments in appreciable amounts.
The presence of barium in a raw mix can reduce the firing temperature from 1450°C to 1400°C and result in increased clinker production. The clinker thus produced would also have an improved mineralogical composition (Timashev and others, 1974). However, Kurdowski (1974) reported only a marginal usefulness of BaO when added in small amounts, stating that it did not significantly affect either the properties of the liquid phase or the rate of lime assimilation; Ba replaced Ca in all clinker phases, except for the ferrite. The optimum BaO concentration was between 0.3% to 0.5%, preferably for clinker containing less flux (silica modulus >3.0) and high C2S content.
Several studies have found Ba to be an effective activator of hydraulicity and strength. The strength obtained from Ba-incorporated clinkers is 10%-20% higher than that of regular clinker of all ages tested under identical conditions (Kurdowski, 1974; Butt and Timashev, 1968; Kurdowski and Szummers, 1968; Kruvchenko and others, 1970; Peukert, 1974).
Beryllium
Beryllium (Be) is present only in traces in the raw materials. It is found only occasionally in the fine fractions of fly ash, an auxiliary material used as a substitute raw material.
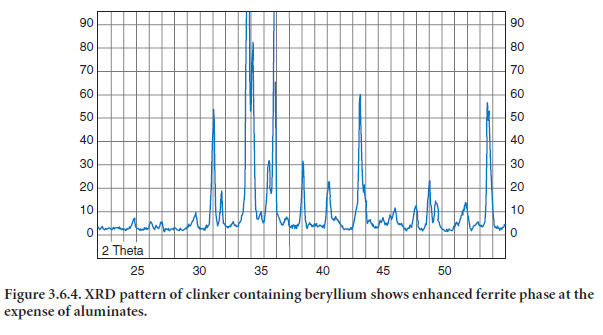
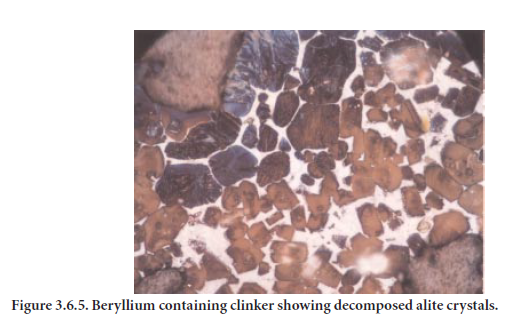
Because of the low volatility of its oxides, beryllium would stay in the clinker. Nonetheless, beryl-lium has not been measured in clinkers at levels sufficient to have any measurable effects on clinker formation or cement use. In a recent study, Bhatty (2003) has noted that beryllium enhances formation of ferrite at the expense of aluminates (Figure 3.6.4). Additionally, alite crys-tals are decomposed (Figure 3.6.5). Beryllium also imparts black color to clinker. The presence of moderate amount of beryllium (about 0.25% by mass) severely affected the setting and strength properties of cement.
Bismuth
Bismuth (Bi) also occurs as a trace element in the raw feed and fuel. The stable oxide Bi2O3 is the least volatile (boiling point 1860°C). Little is available on the influ-ence of Bi in cement manufacturing, but, owing to its low concentration, it is probable that the effects will be practically insignificant.
Boron
Boron (B) is generally found in traces (3 ppm) in most cement raw materials, particularly those containing iron ore.
Kolovos and others (2002) have reported that addition of boron to the raw mix can cause free lime in clinker to increase. According to Mircea (1965), B2O3 decomposes C3S to form C2S, C5BS*, and free lime. Upon further addition of B2O3,C3S completely disappears. Timachev (1980) established a relationship between the electronegativity of boron and the melt viscosity, and noted a similarity between borates, phosphates, and sulfates. Boron inhibited the formation of C3S and affected the stability of the other major clinker phases. In the presence of boron, C3S is decomposed to a stabi-lized C2S as follows:
C3S → b C2S + CaO
It was also pointed out that although B2O3 may not be a useful addition for regular alite clinker required for early strength development, it might be useful as a mineralizer for belitic clinkers. Gartner (1980) has reported on the effectiveness of B2O3 to stabilize ?C2S and to improve its hydraulicity. According to Miller (1976), boron can also stabilize ?C2S in alumina and iron-poor systems. However, it was cautioned that the indiscriminate addition of boron can produce unpre-dictable hydration results. Gartner (1980) argued that this behavior of boron is probably sensitive to the presence of other trace elements.
Bromine
With some exceptions, bromine (Br) plays a minor role in cement manufacturing. Bromine occurs only as a minor element in raw materials, for example limestone (6 ppm), clay (10-58 ppm), and coal (7-11ppm) (Akstinat and Rott, 1988; Sprung, 1985). Bromine has also been detected at meas-urable levels in some of the fly ashes generated at coal-operated power plants.
Bromine is volatile and expected to end up in stack emissions (Akstinat and Rott, 1988). Under oxidizing conditions, bromine gas (Br2) would form and end up in emissions. Retention of bromine in clinker is negligible. Alkali bromide may be found at low concentration in CKD.
Cadmium
Cadmium (Cd) occurs in traces in the raw materials and fuels. Cadmium in the raw feed reacts with the constituent of kiln gas and can form halides or sulfates, both of which are readily vaporized at peak kiln temperature. The form of cadmium incorporated in clinker is not known; however, with increasing chloride input in the kiln, the concentration of Cd in clinker decreases. The addition of CdCl2 to the raw mix has the same effect. In a cyclone preheater kiln, 74%-88% of the total Cd entering the kiln is incorporated in clinker as opposed to 25%-64% for that produced in the grate preheater kilns; the remaining Cd is captured in the kiln dust (Weisweiler and Krcˇmar, 1990).
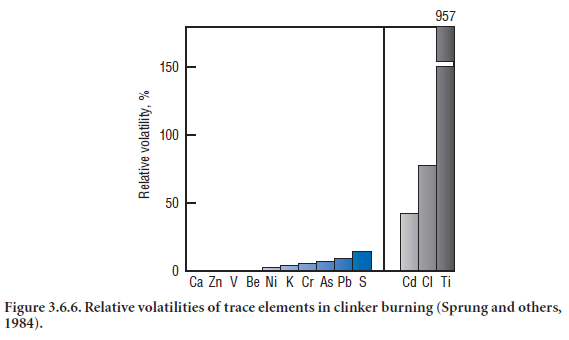
Cadmium is also volatile in nature, although not as volatile as thallium or chlorine. Volatility of cadmium relative to other elements in the kiln feed is shown in Figure 3.6.6 (Sprung and others, 1984). CdO reportedly increased the burnability of the clinker by lowering the melt temperatures whereby Cd2+ most likely entered the silicate phases (Ramankulov and others, 1964). An improve-ment in clinker burnability with CdO addition was also noted by Odler and Abdul-Maula (1980-a).
Calcium
Since the role of calcium (Ca) in cement manufacturing is already well documented, it is not discussed here. However it has been briefly discussed earlier in the section of major elements.
Carbon
Carbon (C) is a major component of fuel. It is also present as carbonate in the limestone. A signifi-cant amount of carbon can also be present in fly ash as unburned coal. Carbon as CO2 is exten-sively present in cement kiln systems, but not in any significant level in clinker. Because of the limestone and fuel used in the kiln, the gases emitted from the stack mainly constitute CO2,H2O, and N2.Limestone (CaCO3) decomposes to CaO and CO2 at about 900°C. For roughly every three tons of clinker, two tons of CO2 are generated and essentially released through the kiln stack.
Cesium
Cesium (Cs) is found only as traces in cement raw mix or in the fuel, generally occurring in cement at 0.02% or less (Blaine and others, 1965). Cesium is expected to form stable sulfates and volatile chlorides in the kiln (Gartner, 1980). On the other hand, its concentrations may be too low to effectively influence clinker formation or cement properties.
Chlorine
Chlorine (Cl) as chlorides is also frequently found in limestone, clays, and sometimes in both primary and secondary fuels. The predominant chloride in limestone and clay is sodium chloride (Akstinat and Rott, 1988). Some coals can contain up to 0.28% Cl, mainly as rock salt.
The formation of stable yet volatile alkali chlorides NaCl (boiling temperature = 1413°C) and KCl(subliming temperature = 1500°C) at clinkering temperature is well known. Both chlorides volatilize in the burning zone and condense in the cooler parts to form kiln rings or preheater build-ups which impair plant performance. Bhatty (1985) also concluded that agglomeration due to the presence of molten alkali chlorides was one of the major reasons for the buildups. In cases of plants without preheaters, the volatile chlorides end up in the kiln dust. In preheater kilns, up to 99% of the chlorides are recaptured by the incoming feed in the calcining zone (Ritzmann, 1971); the concentration of chloride at that point could be extremely high (>1%) compared to that of raw feed (?0.01%). Relative volatility of chlorides and other elements in the kiln system is already shown in Figure 3.6.6 (Sprung and others, 1984).
Wet process plants and grate preheaters may tolerate raw feed with higher chlorides, but the limits primarily depend upon the efficiency of dust collecting and the level of kiln dust recycling. With the advent of the alkali bypass, the chlorine cycle can be broken at the most intense point of kiln circulation, and the alkali chloride can be conveniently concentrated in the dust collectors. Otherwise, as reported by Norbom (1973), a total chloride intake of 0.015% on raw basis (in both raw material and fuel) can result in buildups in a preheater without a bypass.
Since most of the chlorides are volatile, the amount retained in clinker is extremely small (<0.03%). Consequently the alkali level in the clinker is also reduced. The combined influence of alkali chloride on cement properties is therefore regarded as insignificant. In some cases, calcium chloride is added to the kiln for the express purpose of increasing alkali volatilization and removal, and results in the production of a low alkali clinker where dust can be discarded.
In waste-derived fuels such as waste-oils contaminated with chlorides, chlorinated hydrocarbons, and scrap tires, chlorides occur in different forms at much higher concentrations (Akstinat and Rott, 1988) and cause serious operational problems even with a bypass. In order to make the use of such fuels feasible, a large portion of bypass dust needs to be discarded, to eliminate a large chloride cycle.
High chlorides in the raw feed have also been reported to form condensation plumes in the emis-sion stack in long wet or dry kilns which are difficult to remove at times. Such detached plumes are generally the result of NH4Cl formation. Excessive chlorides can also have a deleterious effect on kiln basic brick lining.
Chromium
Chromium (Cr) can be present in raw feed in measurable quantities. Sprung (1985) has reported up to 16 ppm in limestone and nearly 100 ppm in clay and shales. Coals and used oils may contain up to 80 ppm and 50 ppm Cr respectively. Some of the auxiliary raw materials, such as bauxites, which are used up to 4% in cement manufacturing, may contain between 0.04%-0.4% Cr2O3.In addition, a small amount of Cr can also enter cement from the grinding media during raw meal preparation, cement finish milling, and refractory linings.
The presence of Cr in raw materials is known to reduce the viscosity of clinker melt due to its high ionic charge as is shown in Figure 3.6.7 (Timachev, 1980). Miller (1976) has reported improved clinker burnability at 1% Cr2O3 addition. Chromium can exist in a number of oxidation states in clinker, the most stable being Cr3+ and Cr6+.Their formation is sensitive to the oxygen level in the kiln. High oxygen tends to form Cr6+ compounds as chromates which are readily soluble in water and markedly affect the hydration characteristics of the paste. Reducing conditions favor the formation of Cr3+ compounds which are less soluble in mix water.
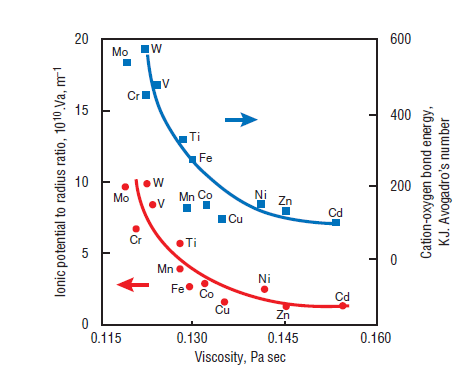
Figure 3.6.7. Relationship between viscosity and ionic potential to radius ratio, and cation-oxygen bond of transition elements at 1450°C (Timachev, 1980).
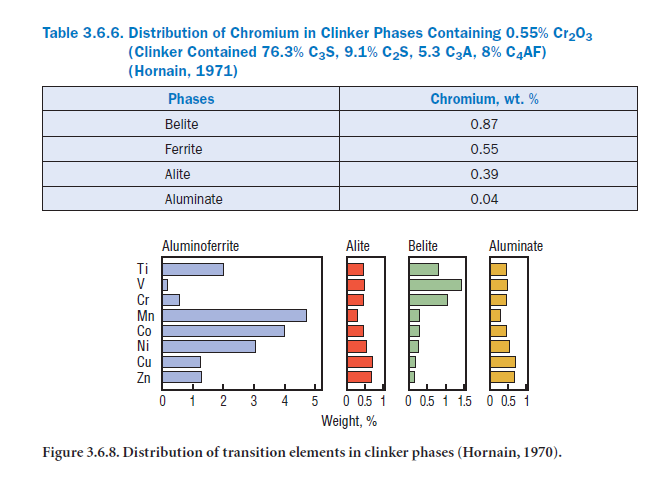
Under oxidation conditions, Cr can also exist as Cr4+ and Cr5+ in C2S, which can then be dispro-portionate to the more stable Cr3+ and Cr6+ upon mixing with water (Feng and Yan, 1990). Johansen (1972) has reported Cr4+,Cr4.6+,and Cr5+ in alite substituting for Si4+.Hornain (1971) reported that Cr preferentially resides in belite followed by ferrite, alite, and aluminates, as shown in Table 3.6.6 (see also Figure 3.6.8). Although Cr6+ can be present in both alite and belite, it is reported to stabilize the B C2S form (Hornain, 1971, and Kondo, 1963). Suba Rao and Narang (1987) developed an active belite-rich cement from raw feed containing 4%-5% Cr2O3 by weight. Imlach (1975) used 0.11%-1.32% Cr2O3 in the raw feed as a flux. The resulting cement exhibited improved 8- and 24-hour strengths, but 28-day strengths always decreased.
A significant portion of Cr can also enter the finished cement from chrome-rich grinding media (Klemm, 1992). It is reported that the level of Cr6+ in ground cement is almost doubled by the use of high chromium alloy balls during finished grinding. A number of patents report the use of inor-ganic reductants to control the Cr6+ leaching from cement. Most of these patents are of European origin and use ferrous sulfate heptahydrate, ammonium-ferrous sulfate, and manganese sulfate during intergrinding to convert Cr6+ to Cr3+,which is more stable in an aqueous environment and is not a significant health risk.
Kolovos and others (2002) have reported that addition of 1% Cr2O3 to the raw mix can cause an increase in free lime of clinker. According to Stephen and others (1999, 2001), however, up to 0.5%addition of chromium lowers the free lime, but 2.5% chromium leads to decomposition of alite to form more belite and consequently more free lime. Chromium is mainly found in belite, but if potassium is high in the clinker, K2CrO4 and/or K2Cr2O7 can also form.
Although a major portion of chromium incorporated in clinker is usually tied up in belite, ferrite, or sulfate phases, chromium oxide is somewhat volatile and can end up in the CKD. Between 100-1000 ppm of Cr have been reported in CKD (Lee and von Lehmden, 1973). Delles and others (1992) have shown Cr only between <0.01-264 ppm in CKDs produced by burning conventional fuels, and between <0.01-299 ppm in CKDs produced by waste fuels. Detectable levels of 20.6 mg/sec and 12.5 mg/sec have also been found in kiln emissions using conventional as well as waste fuels respectively (Mantus and others, 1992). In U.S. cement, total chromium is reported to be between 20 ppm and 450 ppm.
Bhatty (2003) has shown that chromiu
m addition enlarges the alite crystal size. However, at higher chromium contents, decomposition of alite becomes persistent with ragged edges while belite becomes visibly dendritic as already shown in Figure 3.6.1. Chromium also imparts dark green color to clinker.
Cobalt
Cobalt (Co) is present in traces in the raw mix; the maximum reported concentration is 23 ppm CoO. It has also been found at much higher levels (up to 1.27%) in some of the coal fly ashes that could be used as a component of cement raw feed (Bucchi, 1981).
The majority of cobalt that becomes present in the raw mix is incorporated in clinker. The CoO level reported in cement is <130 ppm, but the amounts detected in the alite and belite phases are only traces, since the bulk of cobalt is concentrated in the ferrite phase, replacing Fe3+ and forming the “C4ACo” phase (see Figure 3.6.3).
Cobalt can also give color to cement.
Sychev and Corneev (1964) demonstrated that Co somewhat reduces the hydraulic activity of alite and increases clinker hardness. According to Miller (1976), cobalt increases the water demand and marginally reduces the late strength of cement paste. Cobalt and its oxides are unlikely to vaporize in the kiln or report to the CKD.
Copper
Bucchi (1981) has quoted an average of 16 ppm cupric oxide (CuO) in the raw mixes, and up to 0.13% in coal fly ashes. An average of 90 ppm CuO occurs in commercial clinkers. Copper prefer-entially concentrates in the ferrite phase, followed by alite, aluminate, and belite (see Figure 3.6.3; Hornain, 1971). Miller (1976) reported that under oxidizing conditions, the small amount of copper present as CuO stabilizes alite, whereas under reducing conditions, copper as Cu2O adversely affects both alite and belite phase formations. CuO can also function as a flux, as it decreases the melt temperature considerably (Rumyanstev and Kozlov, 1968). Odler and
Abdul-Maula (1980-a,b) found that 1% CuO addition was effective in reducing free lime at much lower melt temperatures. It may be mentioned that CuO accelerates C3S formation, whereas Cu2O inhibits it. Copper imparts a dark tan color to clinker (Bhatty, 2003).
Kolovos and others (2002) have reported that addition of 1% CuO to the raw mix can reduce free lime in clinker by 30%-60%. Copper oxides are volatile at kiln temperature (melting points CuO=1326°C, Cu2O= 1235°C). Thus, copper has been found up to 500 ppm in some U.S. cement kiln dusts.
Fluorine
Fluorine (F) is commonly present in limestone, clay/shale, and coal (Sprung and von Seebach 1968) as a minor element and plays an important role in cement making. In commercial raw feed, fluorine can be found at up to 0.06% by mass.
Calcium fluoride (CaF2) is also frequently added to raw meal as a mineralizer and flux to lower the burning temperature and accelerate the formation of C3S (Klemm and others, 1976, 1979). However, Miller (1976) has cautioned against using fluoride beyond 0.25% to avoid adverse effects on clinker behavior as it selectively incorporates into the aluminates or silicate phases at certain burning temperatures. At lower temperatures, fluoroaluminates (C11A7 ·CaF2) are formed which decompose at high temperatures to C3A and fluorides. These fluorides are then incorporated into silicates at higher temperatures often forming stable fluosilicates, but excessive amounts can cause decomposition of alite.
Higher levels of alkali can also lead to the formation of alkali fluorides such as NaF and KF (Gartner, 1980). These fluorides, being somewhat volatile (boiling points 1700°C and 1500°C respectively), probably concentrate in the fine kiln dust. However, Sprung and von Seebach (1968) reported that between 88% and 98% of fluorides are incorporated in the clinker, and only a small fraction reports to CKD, probably as CaF2.Fluoride emissions were reported as low (0.009-1.42 mg F–1/Nm3) not necessarily due to the volatility of fluoride, but probably to the efficiency of the precipitators.
Akstinat and Rott (1988) reported that fluorides have no adverse effects on the cement production process, and the fluoride cycle does not cause any operational problems like unwanted coating because of the small quantity involved. However, recent experiences have shown that use of fluo-ride-based compounds can occasionally cause plugging (Bhatty, 1996). The presence of fluorides above 0.5% can, however, cause both operational and quality control problems, which under certain situations can be controlled by P2O5 addition (Gartner, 1980). Goswami and others (1991), Bolio-Arceo and Glasser (1990), and Gilioli and others (1979), have reported the formation of spurrite (2C2S·CaCO3), and fluorellestadite that cause kiln deposits, but the resulting low burning temperatures can control the alkali cycle and reduce the alkali-sulfate deposits.
Palomo and others (1985) noted that 0.2% fluoride promotes low temperature formation of aluminates such as fluorinated C12A7,C3A, and C2AS (gehlenite); however, the final aluminate mineralogy was not significantly affected, as both ferrite and C3A were present at 1250°C and above. According to Perez Mendez and others (1986), with the addition of 0.5%-1.5% fluorides as CaF2, it was possible to complete the clinkering reaction in 30 minutes at 1354°C. Much of the requisite alite was already formed, together with ?C2S, ferrite, and C3A also present therein. Imlach (1974) observed that fluoroaluminate (C11A7·CaF2) forms at fluorine levels of about 0.5%in clinkers fired below 1320°C or slowly cooled from 1340°C to 1265°C. Fluoroaluminate imparts rapid setting to cements (Greening and others, 1971).
The presence of F and Al beyond the threshold level gives C3S a rhombohedral symmetry, which is associated with improved hydraulic properties (Aldous, 1983; Shame and Glasser, 1987). Moir(1983) demonstrated that by optimizing the levels of F, alumina, alkalies, and sulfates, the C3S in clinker could be maximized to enhance the setting properties of cement. An optimum fluoride addition for maximum strength at early ages (24 hours) was 0.2%. For 7-day and 28-day strengths, additions of 0.75% were acceptable.
Gallium
Gallium (Ga) is found only in traces in raw material and coal. Gallium can be present in coal at 5-10 ppm level. Gallium is also sometimes found in coal fly ash. Being relatively volatile in nature, gallium can end up in cement kiln dust.
Germanium
Germanium (Ge) is a trace element found in raw material and coal. As an analogue of silica, germanium oxide (GeO2), is not volatile and is likely to concentrate in clinker. When present in larger amounts, GeO2 can form C3G*(Ca3GeO5) with CaCO3 at 1500°C and is stable between 1335°C-1880°C. At temperatures below 1335°C, C3G decomposes to C2G (Ca2GeO4) and free lime (Boikova and Domansky, 1974). These forms of calcium germanates are similar to C3S and C2S respectively. C3G is hydraulic and produces calcium germanate hydrate (C-G-H) and calcium hydroxide (CH) with water, whereas C2G is assumed to be non-hydraulic. According to Gartner
(1980), it is unlikely that small amounts of Ge would seriously affect the formation of clinker and the properties of the resulting cement.
Hydrogen
The role of hydrogen (H) in cement manufacturing has not been documented in detail because, being highly combustible, hydrogen does not exist in the kiln as hydrogen per se except in trace amounts in the flame as H atoms. It is present in the kiln as water vapor, which results from the evaporation of physically bound moisture from the raw material, and from the evaporation of water sprayed on the raw feed to control dusting. Water vapor is also present in the kiln gases from combustion of fuel as follows:
CH4 + 2O2 → CO2 + 2H2O
A portion of water may also come from the dehydration of raw materials such as clays where it can be present in significant quantities, depending upon their mineralogical nature.
The water vapor present in the kiln probably has an indirect effect on the volatility of alkalies which can increase with vapor pressure at higher temperatures such as:
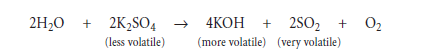
Apart from that, hydrogen is not believed to have any significant effect on the anhydrous nature of clinker.
As a point of information, it may be mentioned that early cement kilns sometimes used “producer gas” as fuel. The gas was generated (as a mixture of CO and H2) by the action of steam on hot coal or charcoal as follows:
H2O + C → CO + H2
Indium
Indium (In) is present only in traces in raw material and fuel; typical concentration of indium in coal is 0.07 ppm. Indium is volatile and ends up concentrated in the kiln dust.
Iodine
The presence of iodine (I) in limestone and clay is negligible: up to 0.75 ppm in limestone, 2.2 ppm in clay and shales, and between 0.8 to 11.2 ppm in coal (Sprung, 1985). Because of the low levels of iodine in the raw feed, the effect on the burning process is negligible. Iodine salts are volatile in nature and mostly end up in dust or emissions. Their concentration in clinker is detected at very low levels.
Iron
Iron is a major element. Since the role of iron in cement manufacturing is well understood, it is not discussed here. However, it is briefly discussed in the section dealing with major elements.
Lead
Lead (Pb) is often present in trace amounts in raw materials – mainly clay and shale. It is present at appreciable levels in coals and others, used oils, lubricating oils, and scrap tires. In fly ash, lead tends to concentrate in the fine fractions (Coles, 1979). Lead levels in coal, used oil, and petroleum coke are shown in Table 3.6.4.
The effect of lead in cement manufacturing and cement properties has been studied in some detail. Lead compounds are fairly volatile. They tend to vaporize in the kiln and exit the kiln as fines, and are collected in the kiln dust. Mantus and others (1992) have shown that the lead concentration levels in the CKD from cement plants burning certain waste fuel (277 ppm) can be much higher than those plants using conventional fuel (83.1 ppm).
There is also evidence that despite the partitioning of lead into the CKD, the bulk of the lead can still be retained in the clinker (Berry and others, 1975). However, this would have no adverse effect on the cement properties if present below 70 ppm. The effect of lead levels higher than that in clinker is uncertain (Sprung and Rechenberg, 1978).
Lithium
Lithium (Li) is found in some waste materials such as used lubricants, but occurs only in traces in typical raw materials and fuels.
Lithium would tend to form a relatively nonvolatile oxide (Li2O) at elevated kiln temperatures. Gouda (1980) reported that Li2O is most reactive in lowering the temperature of the initial liquid phase. Rangarao (1977) pointed out that up to 1% of Li2O in the raw mix imparts a mineralizing effect, but lime fixation is impaired thereafter. At a lower Li2O addition (0.1%-0.3% by weight), limestone dissociation energy is reduced, and mineral formation becomes more intensive; this appears to be a contradiction to the usual behavior of mineralizers and clinker poisons.
Magnesium
Magnesium (Mg) in portland cement is derived mainly from magnesium carbonates present in the limestone in the form of dolomite CaCO3·MgCO3,while smaller amounts usually come from clay and shale (Lea, 1971).
If present in small quantities, magnesium improves the burnability of clinker (Christensen, 1978), although, according to Long (1983), the behavior of MgO in clinker formation primarily depends upon the cooling rate. When clinker is burned at high temperature (>1500°C) and rapidly cooled, it retains most of the MgO in aluminate and ferrite phases, with some in alite as well. But on slow cooling, only 1.5% of MgO is retained in solid solution, and the rest is crystallized as large peri-clase crystals. MgO in cement is usually limited to under 5%, because MgO content in excess of 2%can occur as periclase (Taylor, 1997). Under unfavorable conditions, large crystals of periclase in cement slowly react with water to form expansive Mg(OH)2 and can lead to expansion of concrete. ASTM C150 specifications allow up to 6% MgO in portland cements.
Manganese
Manganese (Mn) in clinker comes both from the primary and auxiliary raw materials. Limestone may contain up to 1.91% Mn2O3 as the carbonate mineral rhodochrosite, whereas shales and bauxite could have up to 58.9% and 36.7% by weight respectively (Bucchi, 1981). Mn2O3 could be present up to 1.2% in blast furnace slag, and 1.44% in coal fly ash.
Cement produced from slags can contain more than 1% Mn2O3,which usually imparts a brown color to cement (Lea, 1971). The polymorphism of silicate in clinker is affected by the presence of manganese oxides in the raw material. Knöfel and others (1984) reported that the limit of Mn2O3 substitution in C3S is approximately 2.2% at 1550°C. At lower concentrations, say ~0.1% Mn2O3, single substitution of Si4+ by Mn4+ takes place, whereas at 2.2% Mn2O3 concentration, a double substitution of Si4+ by Mn4+ and Ca2+ by Mn2+ is possible. The stabilized C3S polymorph was identified as monoclinic; Gutt and Osborne (1969) reported it to be triclinic. Miller (1976) demonstrated that at low levels (<0.7%), Mn stabilizes monoclinic alite, but at high levels and in the presence of fluoride, triagonal alite with markedly high hydraulicity is formed.
Manganese can occur in a number of oxidation states depending upon the burning conditions in the kiln and can impart different colors in clinkers, ranging from reddish-brown to blue. Puertas and others (1988) have studied the influence of kiln atmosphere on Mn solid solutions in C3S and C2S. It was reported that under reducing conditions isomorphous replacement of Ca2+ by Mn2+ occurs, while at higher oxygen levels, Mn4+ replaces Si4+.
Alite content of clinker increases with Mn addition, with maximum alite attained at 0.5% MnO2 and 1% Mn2O3 (Knöfel and Gies, 1983). According to Hornain (1971), high Mn content promotes the formation of belite in the silicate phases, but is preferentially incorporated into the ferrite phase through the formation of “alumino-manganite,” such as “C 4AMn” (see Figure 3.6.8). This reduces C3A and marginally increases free lime, thus reducing the early strength of cement.
Manganese will not volatilize at kiln temperature (boiling point = 1960°C) and is unlikely to concen-trate in the CKD. Manganese at higher levels may impart a dark brown color to clinker (Bhatty, 2003).
Mercury
Mercury (Hg) is a trace element. It is highly volatile and vaporizes at very modest temperatures (boiling point = 557°C). Mercury is somewhat inert, and very little is known about its interaction in the clinker making process. It is very likely that mercury and its compounds would volatilize in the pre-calcination region at temperatures closer to 400°C and escape with the stack gases. Mantus and others (1992) have shown that the emission levels of mercury from cement plants burning waste fuel (2.14 mg/sec) are noticeably higher than those using conventional fuel (0.984 mg/sec). Mercury levels in the respective kiln dusts are the same (0.4 ppm).
Molybdenum
Molybdenum (Mo) is potentially an important trace element in lubricating oil (Gartner, 1980). In coal fly ash, MoO3 could be as high as 1.5% by mass. Up to 0.05% of Mo has been reported in clink-ers (Blaine and others, 1965). Molybdenum oxide, having a small radius and a high charge number, is an effective reducer of the clinker melt viscosity as shown in Figure 3.6.7. Kakali and others (1990) reported the formation of larger and roundish alite crystals with some modified belite in clinker prepared with up to 1.5% MoO3 addition. However, cements made from these clinkers showed no adverse effects on their physical properties (Kakali and others, 1989). Kolovos and others (2002) have reported that addition of 1% MoO3 to the raw mix can reduce free lime in clinker by 30%-60%.
Nickel
Sprung (1985) has reported traces of nickel (Ni) in limestone (1.5-7.5 ppm), clay or shale (61 ppm-71 ppm), coal (20 ppm-80 ppm), used oil (3 ppm-30 ppm), and petroleum coke (208 ppm). In coal fly ashes, NiO may be present up to 1.9% (Bucchi, 1981).
Nickel preferentially concentrates in the ferrite phase, followed by the alite, aluminate, and belite phases, as shown in Figure 3.6.8 (Hornain, 1971). Between 0.5% to 1.0% nickel stabilizes alite
(Rangaro, 1977). NiO substitutes for CaO up to 4 mole % in alite and stabilizes the monoclinic form (Enculeseu, 1974). This alite modification apparently enhances cement compressive strength. Studies by Stephen and others (1999, 2001), has also indicated that nickel becomes incorporated in C3S lattice by replacing calcium. Even high intakes of nickel have only marginal effect on free lime. In clinkers with high magnesium levels, a new compound MgNiO2 could form, whereas the remaining nickel goes to the interstitial phases. Nickel imparts dark brown color to clinker (Bhatty, 2003).
Most Ni compounds are nonvolatile, but owing to the volatile nature of some compounds, mostly Ni(CO)4, nickel could end up in the kiln dust, although Delles and others (1992) have shown a maximum of 50.5 ppm Ni in the CKD. In previous studies, concentrations between 100 and 1000 ppm have been recorded in CKDs from certain U.S. cement plants (Lee and von Lehmden, 1973). An average concentration of 17.3 mg/sec has also been found in the cement kiln emissions using conventional fuel, compared to 11.0 mg/sec using waste fuels (Mantus and others, 1992).
Niobium
Niobium (Nb) is another element found in traces in raw materials. Weisweiler and Krˇcmar (1990) has reported more than 30 ppm niobium in the raw feed of a German plant.
Because of its low concentration in the raw mix, niobium would be expected to have very little effect on clinker formation. Kakali and others (1990) reported a very feeble effect of Nb5+ addition (up to 1.5% by weight) on the mineralogical texture and viscosity of clinker melts because of its low ionic charge to atomic radius ratio. Cement prepared from these clinkers did not show any noticeable change in setting or strength properties when compared to regular cement (Kakali and others, 1989).
Because niobium is a high temperature metal (melting point = 2468°C), it would be unlikely to concentrate in the kiln dust or stack emissions.
Nitrogen
Nitrogen (N) can be present up to 0.01% by weight in the raw materials, but in coal and other fuels nitrogen can be as high as 2%, as hetrocyclic nitrogen compounds.
Clinker made under reducing conditions tends to have up to 0.05% N as nitrides. Under oxidizing conditions, nitrogen in clinker is present at only a few ppm.
High concentration of nitrogen from air, increased residence temperatures, higher oxygen partial pressure in the flame zone, and the subsequent oxidation of nitrogen leads to the formation of several oxides of nitrogen (NO, NO2, and N2O4) collectively known as NOx in the emissions. Total NOx results from fuel-nitrogen NO, thermal NO, and prompt NO. In cement manufacture, fuel NOx and thermal NO play significant role. The prompt NO, which is formed by the participation of CH in the oxidation of nitrogen in air, plays a lesser role (Bretrup, 1991).
The quantity of thermal NO formed is closely related to the burning zone temperature (BZT). According to Lowes and Evans, (1989), a reduction in BZT from 1500°C to 1300°C can reduce the NOx levels by 200-400 ppm. It is not known to what degree the nitrogen in the raw feed con-tributes to NOx emissions. In precalciners, fuel N plays a role, but in the burning zone, the temper-ature is so high that thermal NO is almost in equilibrium; fuel N has little effect (Miller and Egeløv, 1980).
Oxygen
The role of oxygen (O) per se on the manufacture and use of cement has not been studied. Oxygen in the combustion air is needed to combust the fuel. Nonetheless, a considerable portion of raw material and clinker phases incorporate oxygen in one form or the other. Raw material is primarily composed of CaCO3 (?75%), SiO2 (?20%), and Al2O3 (?2%). CaCO3 in the raw mix is derived from limestone; SiO2 and Al2O3 from clays, shales, sandstones, and bauxite; and Fe2O3 from iron oxides, iron ores, and bauxite.
The burning conditions in the kiln can be of an oxidizing or reducing nature. Clinker is typically made in a kiln having a 2%-3% excess oxygen level. Clinker made under oxidizing conditions tends to incorporate trace metals at higher oxidation states as opposed to those prepared under reducing conditions. Examples of chromium and sulfur can be cited here. Cr6+ would form under oxidizing conditions instead of Cr3+,which results from reducing conditions. Cr has also been reported to occur as Cr4+,Cr4.6+,and Cr5+ (Johansen, 1972), but eventually it disproportionates to the more stable Cr3+ and Cr6+ when mixed with water.
Alkali sulfates formed in the kiln may be decomposed under reducing conditions. Kilns with strong oxidizing conditions and low burning zone temperature achieve more sulfur retention in clinker than those produced under reducing conditions and high burning zone temperature. Thus, the oxidation or reducing conditions in the kiln lead to significant phase changes in clinker.
Clinkers produced under reducing conditions are often brownish compared to darker clinkers made under oxidation conditions. Burning conditions may also have an effect on the crystallinity of major phases. The effects can be pronounced if trace metals are also present.
Phosphorus
Phosphorus (P) as phosphates is present in limestone and shale (Moir and Glasser, 1992); it is also present in sandstones, sands, and in detrital clays (Bucchi, 1980). Phosphorus also occurs in the blast furnace slags, electric furnace slags, convector slags, and fly ash; it is found in sewage sludge that sometimes functions as a fuel supplement.
Cement clinker typically contains around 0.2% P2O5.A high P2O5 concentration decomposes C3S to C2S and lime. If P2O5 is present in excess of 2.5% by weight, the retention of free lime takes place (Nurse, 1952). However, by correct proportioning and proper burning, a sound clinker can be produced, although cement hardening becomes slower. Matkovich and Young (1986) reported higher hydraulic activity for ?’C2S stabilized by P2O5 than for the ?C2S.
Addition of hydroxyapatite Ca5(PO4)3 · OH leads to increased free lime at 1300°C, being directly proportional to the P2O5 content (Odler and Abdul-Maula, 1980-a). This effect was attributed to the preferential stabilization of C2S solid solution at increasing P2O5 additions. However, Halicz and Nathan (1984) demonstrated that a satisfactory C3S phase in clinker was formed by adding P2O5 in the raw feed and maintaining lime saturation factor (LSF) and silica ratio (SR) at 1.0 and 2.75 respectively.
In a CaO-C2S-C3P system at 1500°C, a raw mix with more than a few percent P2O5 does not yield C3S. However, in the presence of fluorine, the tolerance to P2O5 is somewhat improved. It is very likely that the thermodynamics of the system favor the fluoride/aluminum C3S solid solution rather than P-C2S solid solution and apparently form a fluoroapatite phase (C10P3 ·CaF2) which is dissolved in C3S (Gurevich and Dobrynina, 1977). Gartner (1980) suggested that chlorides may also help stabilize P2O5 in C3S by forming a stable chloroapatite C10P3 ·CaCl2 which also forms a stable solid solution with fluoroapatite.
An appropriate level of P2O5 in clinker reduces the negative effects of alkali on the strength prop-erties of cements (Coleman, 1992). In cement clinkers with “normal” Na2O contents of 0.8%, the maximum 28-day strength was achieved at the 1.0% P2O5 level.
Potassium
See sodium section for detailed discussion on potassium (K). Since both potassium and sodium (Na) occur together in raw materials and by virtue of similarities in their behavior in cement manufacture, they have been discussed together.
Rubidium
Rubidium (Rb) occurs only as traces in cement raw materials or in fuel; its concentration in cement is generally 0.02% or less (Blaine and others, 1965). Rubidium is expected to form stable sulfates and volatile chlorides during the kiln operation (Gartner, 1980). On the other hand, its concentrations may be too low to influence clinker formation or cement properties.
Selenium
Selenium (Se) may be associated with sulfur in coal, but only in traces. It is also present in fly ash where it tends to concentrate in the fine fractions (Coles and others, 1979).
Selenium is volatile (boiling point = 684°C) and is expected to end up as the oxide (SeO2) in kiln dust or in the emissions. Selenium could form less stable selenates (SeO42–), which are unlikely to remain in clinker. Since their concentration is extremely low in the kiln feed, it is unlikely that they will have any significant effect on the manufacture or properties of cement.
Silicon
Silicon (Si) is a major element. Since the role of Si in cement manufacturing is well understood, it is not discussed here. However it is briefly discussed in the section on major elements.
Silver
Silver (Ag) is present only in traces in both the raw material and coal; in coal it may occur as silver sulfides or as a complex.
Since silver occurs only in traces, it not expected to significantly contribute in the clinkering process. Silver is present at 9.2 ppm in cement; it is reported in CKD at 6 ppm for kilns operated with conventional fuels and at 2.5 ppm for kilns using waste fuels (Delles and others, 1992).
Sodium and Potassium
Since both sodium (Na) and potassium (K) occur together in raw feed, and by virtue of similarities in their behavior in cement manufacture, they have been discussed together in this section.
Sodium and potassium are derived mainly from the raw materials; their main carrier is clay or shale. Sedimentary rocks, including the carbonates, sometimes contain soluble alkali salts. Lea (1971) has quoted the occurrence of Na and K in different raw materials used for cement manufac-turing as shown in Table 3.6.7.
Table 3.6.7. Sodium and Potassium Levels in Typical Cement Raw Mix and Materials,Wt. % (Lea, 1971)
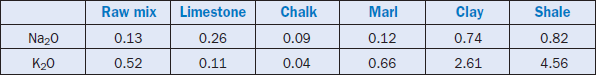
Sodium and potassium also occur in auxiliary raw materials such as blast furnace slag and fly ash as shown in Table 3.6.3. Na2O and K2O in European fuels range between 0.05%-0.6% and 0.5%-2%, respectively (Bucchi, 1980); for typical raw meal, their concentration levels are 0.13% and 0.47%, respectively, as shown in Table 3.6.1; the values shown in Table 3.6.7 are very similar.
The effects of alkalies in cement manufacturing were reviewed in details by Jawed and Skalny(1977, 1978) and Skalny and Klemm (1981). Na2O and K2O are volatile in nature, giving rise to a cycle inside the kiln. The extent of alkali volatilization varies with raw material composition; for instance, volatilization of alkalies in clays is higher than for those in feldspar. Alkalies present in the feed are volatilized at a material temperature from 1400°C-1500°C as the mix nears the burn-ing zone, but condense at cooler parts of the system, such as suspension preheater riser ducts or in chain systems in long kilns. The formation of rings and coatings on kiln lining resulting from this heating-cooling cycle are generally attributed to alkali condensation and reaction with incoming material or refractories. To avoid excessive buildups in the kiln, a percentage of gases may be bled through a by-pass, so that the alkali sulfates and chlorides may be continuously removed and end up in the cement kiln dust (CKD), that is why these CKDs are typically high in alkali contents. Usually potassium compounds are more volatile than the sodium compounds.
The intensity of the alkali cycle depends upon the nature of their presence in raw material and on the processing and type of kiln (Bucchi, 1981). The retention of alkalies is generally higher for high efficiency kiln systems (Lea, 1971). With gas and oil as fuels, the alkalies tend to volatilize more as compared to coal used as a fuel possibly due, at least in part, to the higher hydrogen levels in these fuels, which leads to higher water vapor concentrations in the burning zone.
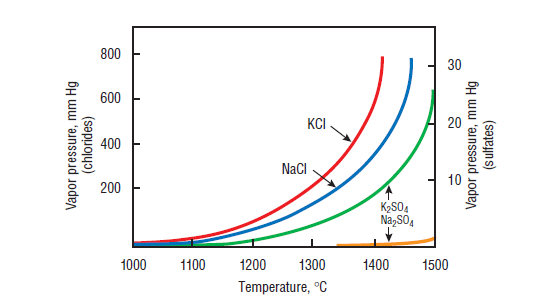
Figure 3.6.9. Vapor pressure of Na and K chlorides and sulfates (Bucchi, 1980).
In the presence of chlorides and sulfate, the volatilization behavior of both Na and K is modified greatly, as shown by the vapor pressure-kiln temperature relationship in Figure 3.6.9 (Bucchi, 1980). Carbonates also have similar effects. In the presence of sulfur, alkalies preferentially form sulfates. If their amount is more than the required stoichiometric balance, the excess will be dissolved in the silicates, aluminates, and ferrites. The common alkali sulfate phases formed are arcanite (K2SO4), sodium potassium-sulfate, also known as aphthitalite of a general solid solution composition (K,Na)2SO4,or more commonly K3NÆ4,and Na2SO4, also known as thenardite (Taylor, 1997).
Burning conditions also significantly influence the formation of double sulfate so that oxidizing conditions produce calcium-potassium sulfate and a reducing condition produces sodium-potas-sium sulfate. Potassium is twice as likely to produce soluble sulfates as sodium. The calcium-potas-sium double salt, calcium langbeinite 2CÆ ·KÆ, is also sometimes found. Thenardite (Na2SO4) is very rare.
The ratio of Na to K in cement raw materials in the North America and Europe varies (Skalny and Klemm, 1981). There is usually a substantial excess of K2O over Na2O. Therefore, in the presence of sufficient amount of SO3,a range of double alkali sulfates, as described in this chapter, is formed depending upon the K2O to Na2O ratio. If K2O is in excess of that required to produce aphthitalite (K3NÆ4), it forms a single sulfate arcanite (K2SO4).
Introducing SO3 jointly with K2O and Na2O into clinker melt leads to phase separation. Since alkalies reduce the melt temperature, and the rate of C3S formation is proportional to the amount of liquid phase, a positive effect on C3S formation could be expected. However, Johansen (1977) reported that C3S, with or without the presence of alkali, has the same amount of free lime after firing at 1400°C -1500°C. Alkali sulfate melt and clinker liquid are immiscible phases. Alkalies inhibit the formation of C3S from C2S and lime, because they stabilize the lower energy C2S phase in the absence of sulfates, it might, however, be pointed out that alkalies that are not balanced by sulfur inhibit alite formation far more strongly than does, for example, potassium sulfate. This may be in part because alkali sulfate melt does not participate in the clinkering reaction, whereas alkali in the clinker melt can enter the belite phase and inhibit its conversion to alite.
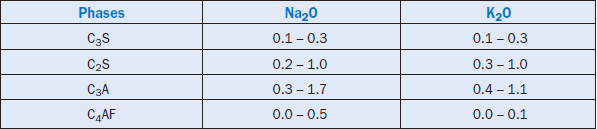
After allocating for the sulfates, the remaining alkalies are distributed between silicates, aluminates, and aluminoferrite. Lea (1971) has reported the following ranges of alkalies in the major clinker phases (see Table 3.6.8). The values are in general agreement to those quoted by Taylor (1997) on the distribution of alkalies in different clinker phases.
Table 3.6.8. Alkalies Concentration in Typical Clinker, Wt. % (Lea, 1971)
Gartner (1980), and Gies and Knöfel, (1986) reported that in the absence of SO3,Na2O is preferen-tially incorporated in C3A by replacing CaO and forms “alkali-aluminate” of an approximate composition NC8A3, thereby reducing its reactivity. This results in clinkers rich in free lime and aluminate, and can reduce the burnability. Potassium is substituted in C2S as a compound with an approximate composition of KC23S12, and the overall reactivity of clinker is decreased due to the slower reaction with CaO to form C3S in the burning process. In the presence of sulfate, K2O increases the C3A reactivity (Strunge and others, 1986). Richartz (1986) reported that SO3 reduces the extent of alkali solid solution in C3A and hence the reactivity, but improves the cement proper-ties. It might be mentioned that although alkalies, Na2O in particular, may act as fluxes, they are technically less desirable compounds than many of the minor compounds (Bucchi, 1980).
If present in excess, alkalies often lead to higher pH and better early strength, but lower later strengths. They are not desirable because of the deleterious alkali-silica reaction (ASR) with reac-tive aggregates that leads to expansive reactions and can cause serious cracking in concrete. The deleterious effects of alkalies on the mechanical properties of cement may be reduced by gypsum addition to the raw feed, because of the possible elimination of solid solutions of alkalies with clinker minerals (Butt and others, 1971). One possible speculation derived from microscopical studies by Prout (1985), is that gypsum would either increase the volatilization, or eliminate NC8A3 or KC23S12 formation. The latter explanation is probably more likely. According to Lokot and Azelitskaya (1969), the addition of gypsum to raw feed produces cement of high 28-day strength, enhancing kiln output and fuel savings.
Strontium
A major portion of strontium (Sr), found in clinker as SrO, comes from limestone and aragonite. Strontium frequently occurs in clinkers between 0.05%-0.4% (Blaine and others, 1965). The mean value quoted by Moir and Glasser, (1992) in Table 3.6.5 is 0.059%. Brisi and Appendino (1965) and Gilioli and others (1972), demonstrated that small amounts of SrO favor alite formation, but at 4%-5% addition, Sr preferentially distributes in belite rather than alite, and inhibits the formation of alite. Phase equilibrium studies indicate that it also favors free lime formation, with SrO prefer-ably going into solid solution and displacing CaO from other compounds. The tendency of free CaO release during clinkering makes SrCO3 more adverse than SrSO4, and the clinkers having a high lime saturation factor (LSF) may be more vulnerable to free lime expansion during hydration.
Sulfur
Sulfur (S) is frequently present in coals and some fuel oils; sulfates and elemental sulfur are also often present in the limestones. Clayey sediments, marls, also contain both sulfides and sulfates. Locher and others (1972) have reported occasional use of gypsum and anhydrite as mineralizers and modifiers of the alkali cycle in the kiln.
Sulfides and sulfur from raw materials and fuel are oxidized and are incorporated into the solid phases as sulfates in the clinker, though some sulfur as SO2 will almost always escape with the exiting gases. Sulfur is a volatile substance and its behavior in kiln is a complex one. Depending upon the burning conditions in the kiln, both oxidized and reduced species may occur in solid, molten, and vapor phases, as explained by Choi and Glasser (1988) in Figure 3.6.10.
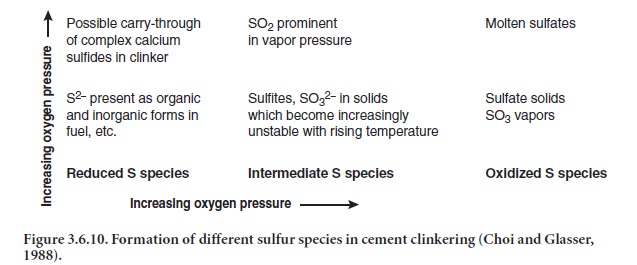
Under oxidizing conditions at high temperature, formation of SO2, and subsequently of SO3, is most likely. In the presence of lime, SO3 is partly removed to form CaSO4 by the following interactions:
SO2 + CaO → CaSO3
CaSO3 + 1⁄2O2 → CaSO4
In the presence of alkali, alkali sulfates are formed which are later condensed at the lower tempera-ture regions and may cause plugging in the preheater. Intermediate compounds such as “sulfos-purrite,” 2C2S·CÆ , and the ternary compound “sulfoaluminate,” C4A3Æ may form rings or also contribute to preheater build-up problems.
Another well known problem of the volatility of sulfur is its cycle of vaporization and condensa-tion with alkalies. They are volatilized at high temperatures and subsequently condense on the relatively cooler incoming raw feed, resulting in high S and alkali levels in the middle zone of kiln, especially with preheaters. The use of an alkali by-pass is often effective to break this cycle and lead to the reduction of S and alkalies in the incoming raw feed. However, alkali sulfate levels are signif-icantly increased in the by-pass dust, which must generally be discarded.
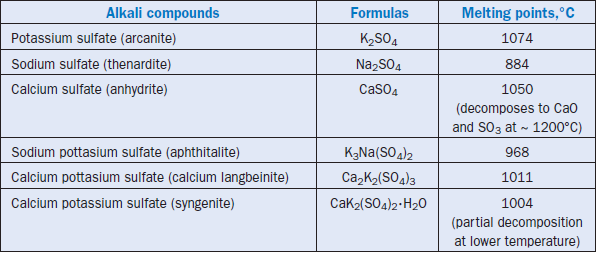
Sulfates (SO3) preferably combine with alkalies to give double alkali sulfates, (K,Na)2SO4,which most probably occur in clinker as K3Na(SO4)2, known as aphthitalite, or K2SO4 known as arcanite. If SO3 is present in excess, the balance between alkali is achieved by forming a double salt known as calcium langbeinite, Ca2K2(SO4)3,which forms a melt at 1011°C in a CaSO4–K2SO4 system. However, this phase is known to evaporate incongruently at high temperatures, and vaporizes K and S (Arceo and Glasser, 1990).
Major alkali salts formed with sulfates and their approximate melting temperatures according to Gartner and Tang (1987), and Skalny and Klemm (1981), are given in Table 3.6.9.
Table 3.6.9. Alkali Sulfates Formed During Clinkering and Approximate Melting Points
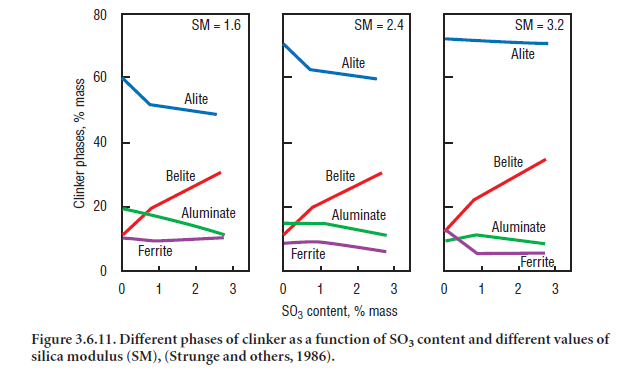
Increasing SO3 contents distinctly decreases alite, and increases belite in clinker; the aluminates and ferrite contents are unchanged in clinkers irrespective of their silica modulus (SM) values (Strunge and others, 1985). On the other hand with increasing SM, irrespective of the SO3, the alite contents are higher, belite is unchanged, and aluminates and ferrite are somewhat lower. Relationships between clinker phases and SO3 content in the clinker as derived by Strunge and others (1986), are shown in Figure 3.6.11. With increasing SO3 contents, the alite crystals in clinker grow larger, and the tendency of belite inclusion in alite is progressively reduced. The crystal size of aluminate and ferrite phases are also significantly reduced.
Gies and Knöfel, (1986, 1987) reported the development of a belite-rich cement by using increased SO3 contents in alkali free raw materials; this clinker showed reasonable hydraulic activity which was attributed to the presence of 0.6%-0.8% SO3 in belite. The rate of clinker cooling did not have any significant effect on the strength properties of resulting cement pastes.
Another related concern is the level of SO2 in kiln emissions. Very frequently, 15%-40% of pyritic (sulfide) sulfur in raw material is converted to the SO2 in the emissions (Neilson, 1991), and has the potential of exceeding SO2 emission limits of 400-800 mg/Nm (dry) specified for European cements.
It may be pointed out that in the preheater system, much of the SO2 in the kiln is taken up by the incoming raw material. However, SO2 may still escape if its concentration is high, or if reducing conditions are generated locally. This often occurs when waste fuels (rubber tires or plastics) are fed into the riser duct (Nielson, 1991). This problem can be minimized by having an excess of combustion air.
Tantalum
Tantalum (Ta) is present only in traces in cement raw materials. It is reported to be present at <9 ppm in raw materials and 0.3 ppm in the oil coke used as fuel in cement (Weisweiler and Krˇcmar, 1990). Since tantalum is present as trace in both the raw feed and fuel, it is unlikely to impart any noticeable effect on clinker formation or cement use. Weisweiler and others (1990) have reported 14.3 and 3.3 ppm tantalum respectively in clinker and kiln dust prepared from a raw material containing 8.9 ppm tantalum.
Kolovos and others (2002) have reported that addition of 1% Ta2O5 to the raw mix can reduce free lime in clinker by 30%-60%.
Tellurium
Like selenium, traces of tellurium (Te) could be associated with sulfur in coal.
At optimum kiln temperature, tellurium can be volatile depending upon its form (amorphous form boiling point = 990°C; rhombohedral form boiling point = 1390°C). Tellurium may form unstable tellurates in clinker and end up in CKD or emissions (Gartner, 1980).
Tin
Tin (Sn) is a trace element in both the raw feed and fuel. Elemental tin is reasonably nonvolatile (boiling point = 2265°C). Tin oxide (SnO) or natural cassiterite melts at 1630°C and sublimes between 1800°C and 1900°C. It is very likely that tin will remain in the clinker. The presence of very small amounts of tin in clinker should not affect cement properties, although not much is known about the effect of tin in clinker manufacture.
Thallium
Thallium (Tl) is found only in traces in raw material; its concentration in coal is only 1.1 ppm. Thallium may be also present in some pyritic minerals used as an iron source for raw feed; it is sometimes found in coal fly ashes, or is present in industrial byproduct iron sources.
Although thallium occurs in traces in the raw feed, it is the most volatile element after mercury in the kiln (melting point = 303°C), and is most likely to concentrate in the kiln dust. The volatility of Tl relative to other elements in the kiln is shown in Figure 3.6.6 (Sprung and others, 1984). Since thallium may be present in the fly ash used in cement raw feed, or as Sprung discovered, in indus-trial byproduct iron sources, it tends to build up in extremely large internal cycles in cement kilns and may lead to greater than 10,000 ppm content in CKD if no dust is discarded.
Titanium
Titanium (Ti) as oxide may be present in typical cement raw material at the 0.02%-0.4% level by weight (Bucchi, 1980). Gartner (1980) reported a higher concentration of 0.1%-1.0% TiO2 in most raw mixes. Concentrations of TiO2 in some of the auxiliary raw materials is even higher. Blast furnace slag, for instance, can contain 1.7% TiO2,and bauxites may have between 2%-8%TiO2 by weight.
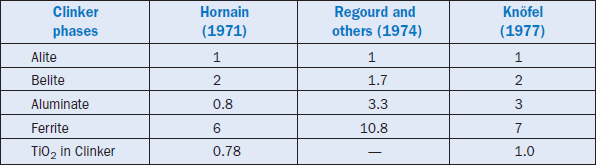
TiO2 is a refractory material (boiling point = 2500°C -3000°C) and is essentially incorporated in clinker. At low levels the effects of Ti on the manufacturing of cement are insignificant (Miller, 1976); higher levels of up to 2% may improve the compressive strength of clinker (Knöfel, 1979). Hornain (1971), and Marinho and Glasser, (1984) reported that TiO2 is preferentially distributed in the ferrite phase. Distribution of other selected transition elements in different clinker phases as determined by Hornain (1971), is shown in Figure 3.6.8. Relative ratios of TiO2 distribution in clinker phases as reported by different workers is also given in Table 3.6.10 for comparison. Titanium additions to kiln feed from ilmenite (FeTiO3) have been used to produce a buff-colored cement. Kolovos and others (2002) have reported that addition of 1% TiO2 to the raw mix can reduce free lime in clinker by 30%-60%.
Knöfel (1977) observed a sharp reduction in alite with equivalent increase in the belite phase when TiO2 was increased in the raw mix; the variation in ferrite and aluminate was not significant. Calcium titanate (CaTiO3) is apparently the major phase present in clinker. It was also reported that about 1% TiO2 addition in the raw mix reduces the melt temperature by 50°C-100°C, proba-bly because of a favorable relationship between ionic potential and the melt viscosity as shown in Figure 3.6.7. This relationship was developed by Timachev (1980). It shows that increasing the ionic potential of transition elements decreases the clinker melt viscosity.
Table 3.6.10. Relative Ratios of TiO2 in Different Clinkers Phases
Bhatty, (2003) noted visible decomposition of alite crystals, and belite with ragged edges in clink-ers containing Ti. Presence of Ti also showed enlargement of interstitial phases (Figure 3.6.12).
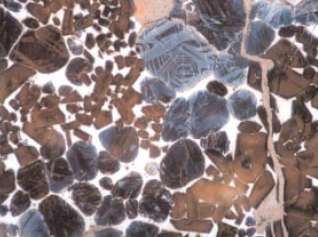
Figure 3.6.12. Decomposed alite and ragged belite along with enlarged interstitials are shown in clinker containing titanium.
Tungsten
Tungsten (W) is a trace metal in the raw mix and is expected to appear in traces in the clinker. Kakali and others (1990) noted that the addition of up to 1.5% WO3 in the raw mix changed the shape of alite crystals, making them bigger and more roundish; the belite formed was of Type III and, to some extent, contained secondary dendritic crystallization, probably because of excessive Si4+ replacement by W6+.Dissolution of W6+ in the melt decreased the viscosity because of its large charge to radius ratio, as is also exhibited in Figure 3.6.7.
Kolovos and others (2002) have reported that addition of 1% WO3 to the raw mix can reduce free lime in clinker by 30%-60%. Ivashchenko (1991) reported that addition of W6+ also improved the granulometric composition of clinker and decreased dusting. Improved hydraulicity was expected because of enhanced activity of ferrite and alite modification in the clinker. However, cement pastes prepared from these clinkers did not show any significant change in the setting or strength properties when compared to regular cements (Kakali and others, 1989).
Tungsten is a very high temperature melting metal (melting point = 3410°C). Since it will not volatilize at kiln temperatures, partition of tungsten to the CKD is highly unlikely.
Vanadium
Vanadium (V) occurs at a measurable level in cement raw material (10-80 ppm in limestone, 98-170 ppm in clay/shale, and 30-50 ppm in coal) (Sprung, 1985). It is also present in fly ash where it tends to concentrate in fine fractions (Coles, 1979). Fairly high levels of vanadium are also reported in crude oils (Gartner, 1980). Weisweiler and Krˇcmar (1990) reported nearly 800 ppm vanadium in some petroleum cokes used in cement manufacturing. Ash from petroleum coke also contains very high levels of V2O5 (up to 60%). But the low overall ash content in the petroleum coke contributes only up to 0.08% V2O5 in clinker produced in modern cement plants that use 50% petroleum coke as a substitute for regular fuel (Moir and Glasser, 1992).
Use of vanadium is known to decrease the melt viscosity primarily due to its higher ionic poten-tial. Vanadium is present as V2O5 in cement clinker. It concentrates in alite and forms larger crys-tals. However, according to Hornain (1971), vanadium preferably resides in belite rather than alite, as shown in Figure 3.6.3. V2O5 (decomposition point = 1750°C) is unlikely to vaporize at normal kiln temperatures. On the other hand, vanadium present in fuel does not have adequate contact with the reacting mass in the kiln and largely ends up in CKD or the gas dust as suggested by Weisweiler and Krˇcmar (1990).
Presence of 1% V2O5 can significantly reduce the free lime in clinker when fired at 1200°C (Odler and Abdul-Maula, 1980-a). V2O5 has also been used for stabilizing ?C2S in clinker apparently by substituting VO43– for SiO44–.According to Bhatty, (2003), presence of vanadium produces belite with ragged edges, Figure 3.6.13. Vanadium also imparts tan color to clinker.
A concentration of 1.5% V2O5 is reported to increase hydraulicity of alite; however, higher concen-trations adversely affect the grindability of resulting clinkers. High V2O5 levels could also deterio-rate kiln lining in some cases (Gartner, 1980). V2O5 in clinker can also increase sulfate expansion under certain circumstances.

Figure 3.6.13. Ragged belite crystals in clinker containing vanadium.
Yttrium
Isomorphism between yttrium (Y) and calcium frequently occurs in natural materials; for instance fluoroapatite, Ca2Ca3(PO4)F, can contain up to 10.6% Y2O3 (Povarennykh, 1966). But presumably in cement raw materials, yttrium occurs only in traces.
Yttrium substitutes for Ca in both C3S and C2S (Boikova, 1986). It yields both triclinic and mono-clinic forms of C3S. In a C2S-Y4(SiO4)3 system, the region of homogeneity can exist up to 35%Y4(SiO4)3 by weight (Toropov and others, 1962-b, 1963).
Since yttrium is unlikely to volatilize at kiln temperature (melting point = 1522°C), it can hardly be expected to concentrate in the kiln dust.
Zinc
Zinc (Zn) is a trace element in the raw mix, comprising 22 ppm-24 ppm in limestone, 59 ppm-115 ppm in clay or shale, and 16 ppm-220 ppm in coal. However, it can be present at up to 3,000 ppm in used oil as a potential secondary fuel, or 10,000 ppm in used automobile tires. Its concen-tration is also reported to be significant in alternative raw materials such as metallurgical slags (Miller, 1976).
Between 80%-90% of the ZnO in the raw mix becomes incorporated in clinker (Sprung and Rochenberg, 1978; Knöfel, 1978). Approximately half of the zinc is distributed in silicates with preference for alite over belite; the other half is distributed into the matrix with preference for the ferrite phase (Knöfel, 1978; Tsuboi and others, 1972). According to Hornain (1971), zinc in clinker is preferentially retained in ferrite followed by alite, aluminate, and belite (Figure 3.6.3). ZnO addi-tions accelerate clinker formation, as would be expected from the relative levels substituted in the alite and belite phases. Formation of alite and C2(AF) is enhanced at the expense of belite and C3A due to ZnO doping (Odler and Schmidt, 1980-c).
Stevula and Petrovic (1981) prepared a triclinic modification of C3S of the type TI-TII from mixtures of 0.75%-1.5% ZnO and pure C3S, fired at about 1600°C, and slowly cooled. Addition of 3.0% and 4.5% ZnO formed rhombohedral C3S with no free ZnO. Boikova (1986) noted changes in C3S symmetry from triclinic to monoclinic and to rhombohedral with increasing ZnO addi-tions. Stephen and others (1999, 2001) also noted that that zinc becomes incorporated in C3S lattice by replacing calcium.
Up to 1.0% ZnO in the raw mix decreases free lime considerably, retards the hydration, and reduces strength when added in excess of 1.0% (Odler and others, 1980-a,b,c). Similar observa-tions were reported by Knöfel (1978). Miller (1976) suggested the likelihood of reducing levels of Zn in clinker by preferentially vaporizing it where the liquid phase is low, thus reducing the poten-tially deleterious effects on cement setting.
As stated earlier, according to Sprung and Rochenberg (1978), the volatility of zinc in preheater kilns could be 10%-20%. For a multi-stage preheater kiln, the capture of ZnO would be more effective and could result in the total incorporation into the clinker. An average of 149 ppm zinc is reported in the CKD from the U.S. plants using conventional fuel and 150 ppm for those using waste fuel (Delles and others, 1992); the corresponding zinc in the emissions are only 2.97 mg/sec and 1.53 mg/sec, respectively.
Zirconium
Zirconium (Zr) is concentrated mostly in siliceous ores used as a component of kiln raw feed (Miller, 1976). Blaine (1965) reported about 0.5% zirconium, in the fully oxidized form of ZrO2,in U.S. clinkers. Kakali and others (1990) found no significant change in the burning and cooling con-ditions for clinker prepared with 0.73%-1.45% Zr2O3; the principal phases, alite, belite, aluminate, and ferrites, were satisfactorily crystallized. However, Zr2O3 changed the size and shape of alite, while belite was modified. Zr2O3 also imparted a noticeable color change to clinker.
A significant retarding effect and a subsequent delay in strength for cements prepared with ZrO containing raw mixes was also reported (Kakali and others, 1989). However, earlier studies by Blaine and others (1965) indicated that smaller ZrO additions increased the early strength of cement.
The Rare Earths
Elements 51-71 in the periodic table are commonly known as “the rare earths” or “lanthanides.” They are present only as traces in raw materials and cement clinkers. Owing to their presence at extremely low levels, they have not been the subject of extensive studies in cement manufacturing. Since the rare earths have very similar properties to one another, it is assumed that they all will have somewhat similar effects on the clinker formation. The following section discusses the find-ings from reported literature data.
Boikova and others (1964, 1966, 1986) and Toropov and others (1962-a,b, 1963) observed the substitution of rare earths for Ca in both C3S and C2S. The solid solution of C3S with oxyorthosili-cates of lanthanum (La) and scandium (Sc) results from the similarities in ionic size, and chemical properties between Ca, La, and Sc. Formation of C3S solid solution with gadolinium (Gd) and neodymium (Nd) have also been reported. Jantzen and Glasser (1979) reported that nearly 15% of Nd4·Si3O12 could be dissolved into ?C2S, which has identical hydraulicity to that of regular ?C2S. La stabilizes all modifications of C3S solid solutions (Stevula and Petrovic, 1981; and Sinclair and Groves, 1984). Gd gives the triclinic and monoclinic formulations, whereas Sc produces only the triclinic C3S solid solutions. Leaching of Nd stabilized in cement pastes is also fairly low.
Lanthanum also stabilizes the solid solution of C2S by substituting for Ca2+ (Toropov and others, 1962-a,b, 1963). Based on the observations on select rare earths such as La, Nd, Gd, and Sc, Boikova (1986) assumed that the remaining rare earths, with similar chemical and ionic characteristics, would isomorphously substitute for Ca2+ in C3S and C2S. As a result, a preferential distribu-tion of rare earths in wastes could be expected in the clinker silicate phases.
Rumyantsev and Skotinkova (1970) reported that the addition of La2O3 in the raw mix accelerates the formation of clinker under laboratory conditions; the engineering properties of the resulting cements are also enhanced when compared to control.
Studies of rare earths in cement manufacturing also stemmed from the possibility of using low to medium level radioactive wastes that might contain significant amounts of rare earth elements. Studies by Jantzen and others (1979, 1982), and Boikova (1986) indicated that raw mixes with 20%-30% radioactive waste composed of La2O3,UO3,Ce2O3, and other oxides, give optimum elemental distribution in clinker when fired at 1100°C-1200°C. This prevents volatilization and activation of radionuclides which occurs at 1200°C. On the other hand, full radionuclide partition-ing of raw mixture with radioactive wastes will not occur at temperatures below 1100°C.
Jantzen and Glasser (1979) developed clinkers by incorporating 15%-20% by weight of radioactive wastes and firing at about 1200°C. The clinkers were slow to react, but the ultimate hydration products were primarily stable insoluble calcium silicates hydrates with reasonable distribution of the radionuclides. Sichov (1968) reported that La, Nd, and Ce enhanced alite hydraulicity.
Rare earths have low volatilities (Klein and others, 1975) and are very unlikely to end up in the kiln dust.
CONCLUDING REMARKS
Although several minor elements have been studied in some detail for their role in the clinkering process and cement properties, data on other elements, especially those found frequently in wastes, are available only to a limited extent. It appears that with the current trend, using raw materials incorporating wastes or by products and burning supplementary fuels with a potential of trace element contents, a need to understand the effects of trace elements on cement manufacturing is paramount. If, with the use of alternative materials and fuels, the chemical make up of the raw mix is not favorable, it may have adverse operational and quality control consequences. Although some progress has been made in understanding the roles of these elements on clinker formation and prop-erties, their interaction with the major clinker components still needs to be properly understood.
The possible source of minor elements from the literature data presented here is summarized in Table 3.6.11, whereas the potential effects of the elements on cement manufacturing as discussed in this chapter are summarized in Table 3.6.12.
Use of some selected trace elements as fluxes or mineralizers to enhance the clinkering process is also being realized, yet understanding of the underlying physico-chemical mechanism, potential
Table 3.6.11. Possible Sources of Minor Elements in Cement Manufacture
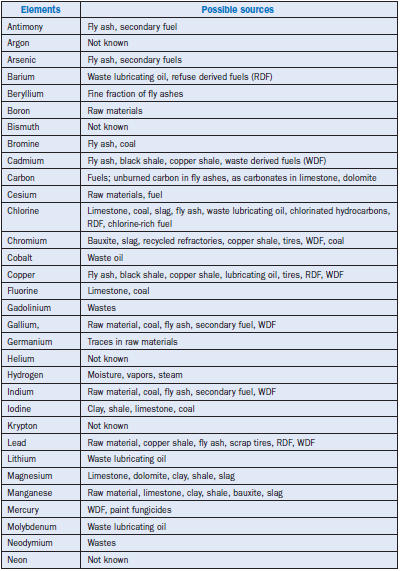
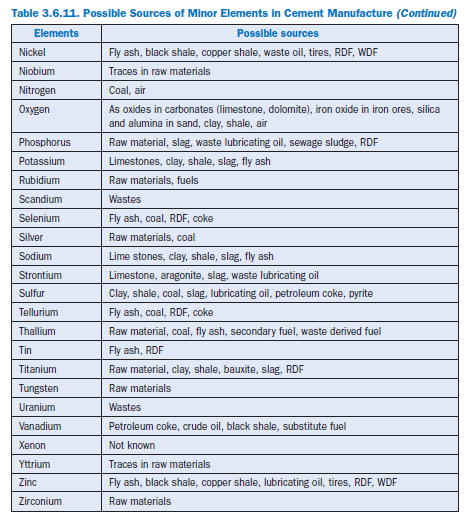
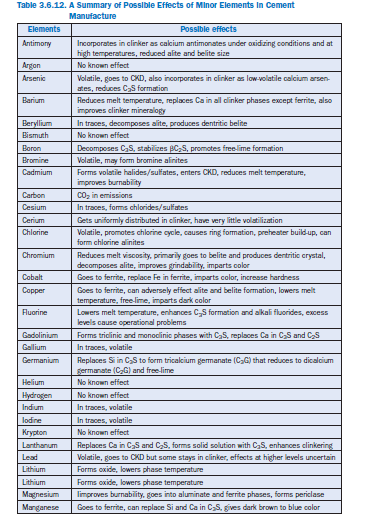
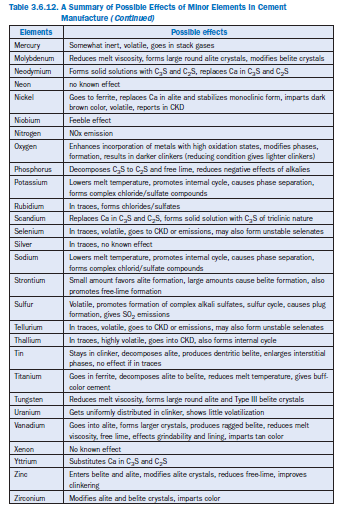
operational advantages, and energy conservation needs to be looked into. Efforts can be directed toward exploring the following aspects of clinker-minor element interaction:
• Make use of the minor elements present either in natural or secondary raw materials and fuels, to enhance the clinkering process and the performance of cement
• Make use of the minor elements in conserving energy during clinker production; elements with proven fluxing/mineralizing characteristics could be targeted for investigation
• Recycle the minor element-bearing secondary fuels that least affect the environments; use of chlorinated hydrocarbons and waste fuels can be the typical examples
REFERENCES
Akstinat, M. H., and Rott, Chr., “Coulometric Determination of Low Halide Concentration in Inorganic Binders and Minerals Raw Materials,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, No. 3, 1988, pages 138-143.
Aldous, R. T. H., “The Hydraulic Behavior of Rhombohedral Alite,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 13, 1983 pages 89-96.
Arceo, H. B., and Glasser, F. P., “Fluxing Reactions of Sulphate and Carbonates in Cement Clinkering. I: Systems CaSO4-K2SO4 and K2SO4-CaCO3,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 20, No. 6, 1990, pages 862-868.
Berry, E. E.; MacDonald, L. P.; and Skinner, D. J, “Experimental Burning of Waste Oil as a Fuel in Cement Manufacture,” Water Pollution Control Directorate, Environment Protection Service, Department of the Environment, Canada, Report EPS-4-WP-75-1, June 1975.
Bhatty, J. I., “Role of Miner Elements in Cement Manufacturing and Use,” Portland Cement Association, Skokie, Illinois, U.S.A., PCA R&D Bulletin RD109T, 1995, 40 pages.
Bhatty, J. I., “Use of Fluxes and Mineralizers in Cement Industry: A Survey,” Portland Cement Association, Skokie, Illinois, U.S.A., PCA R&D Serial No. 2045, 1996.
Bhatty, J. I., “Effect of Minor Elements on Clinker and Cement Manufacture: A Laboratory Analysis,” Portland Cement Association, Skokie, Illinois, U.S.A., PCA R&D Serial No. 2147, 2003.
Bhatty, Muhammad S. Y., Prevention of Build-Ups in Cement Kiln Systems, R&D Serial No. 1735, Portland Cement Association, Skokie, Illinois, U.S.A., June 1985.
Blaine, R. L.; Bean, L.; and Hubbard, E. K., “Occurrence of Minor and Trace Elements in Portland Cement,” Building Science Series #2, National Bureau of Standards, U. S. Department of Commerce, Washington, D.C., U.S.A., August 1965, pages 33-36.
Boikova, A. I., and Torpov, N. A., “Study of Solid Solutions of Tricalcium Silicates with Rare-Earth Oxyortho Silicates,” Experiment in Technical Mineralogy and Petrography, Moscow, U.S.S.R., 1966, pages 25-30.
Boikova, A. I., and Domansky, A. I., “High Temperature Transformation of Pure Calcium Germanates,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 4, No.5, 1974, pages 773-784.
Boikova, A. I.; Toropov, N. A.; and Kuznetsov, A. K., “Rare Earth Silicates as Crystal, Chemical Indicators, Solid Solutions of Tricalcium Silicates with Lanthanum Oxyorthosilicate,” Doklady Academii Nauk SSSR, New York, U.S.A., Vol. 156, No. 4, 1964, pages 865-868.
Boikova, A. I., “Chemical Composition of Raw Materials as the Main Factor Responsible for the Composition, Structure and Properties of Clinker Phases,” 8th. International Congress of Chemistry of Cement, Rio de Janaro, Brazil, Vol. 1, 1986, pages 17-33.
Bolio-Arceo, H., and Glasser, F. P., “Formation of Spurrite, Ca4(SiO4)2CO3,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 20, 1990, pages 301-307.
Bozhenov, P. I.; Kamusher, E. D.; and Glibina, I. V., “Means of Obtaining Boron-Containing Binders and Mortars,” Chemical Abstracts, 56 (7), 69141, April 2, 1962.
Bretrup, L., “NOx Reduction in the Cement Industry by Application of Multi-Stage Combustion (MSC) and Selective Non-Catalytic Reduction (SNCR) Techniques,” International Cement Conference, Cemtech, Prague, Czechoslovakia, April 1991, pages 219-240.
Brisi, C., and Appendino, P., “Equilibrfi allo Stato Solido nel Sistema CaO-SrO-SiO2,” Annali di Chimica, Rome, Italy, Vol. 65, 1965, page 1213.
Bucchi, R., “Influence of the Nature and Preparation of Raw Materials on the Reactivity of Raw Mix,” 7th. International Congress of Chemistry of Cement, Paris, France, Vol. I, 1980, pages I-I/3-43.
Bucchi, R., “Feature on the Role of Minor Compounds in Cement Clinker – Part 1,” World Cement Technology, London, U.K., June 1981, pages 210-231.
Butt, Yu M., and Timashev, V. V., “Technological and Physical-Chemical Characteristics of the Manufacture of Rapid Hardening and High Strength Cements,” Proceedings of 9th. Conference on Silicate Industry (Silicof), Budapest, Hungary, 1968, page 217.
Butt, Yu M.; Turetsky, A. M.; and Panina, N. S., “Effect of Gypsum on Properties of Alkali-Containing Cement,” Tsement, Leningrad, U.S.S.R., Issue 4, 1971, pages 14-16.
Choi, Gang-Soon, and Glasser, F. P., “The Sulfur Cycle in Cement Kilns: Vapor Pressures and Solid-Phase Stability of the Sulfate Phases,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 18, 1988, pages 357-374.
Christensen, N. H., “The Effects of Magnesia on Lime Combination in Clinker,” World Cement Technology, London, U.K., Vol. 9, No. 7, 1978, pages 223-226.
Coleman, T., Unpublished report, Blue Circle Industries PLC, U.K., 1992.
Coles, D. G.; Ragaini, R. C.; Ondov, J. M.; Fisher, G. L.; Silberman, D.; and Prentice, B.A.,
“Chemical Studies of Stack Fly Ash from a Coal-Fired Power Plant,” Environmental Science and Technology, Washington, D.C., U.S.A., Vol. 13, No. 4, October 1979, pages 455-459.
Czamarska, D., “Influence of Some Cations on the Rate of Formation of C3S at 1450°C,” Cement-Wapno-Gips, Warsaw, Poland, Vol. 21/33 (4), April 1966, pages 93-98.
Delles, J. B.; Kanare, H.; Padiyara, S.; and Broton, D., An Analysis of Certain Trace Metals in Cement and Kiln Dusts, SP110.01, Portland Cement Association, Skokie, Illinois, U.S.A., 1992.
Enculescu, M., “Influence of Oxides of Transition Elements on the Properties of Mineralogical Components of Clinkers,” 6th International Congress on the Chemistry of Cements, Moscow,
U.S.S.R., Supplementary Paper I-3, 1974.
Feng Xiuji, and Yan Peiyu, “Effect of the Stages of Chromium Ion on the Colour Characteristics of Doped ?C2S,” Advances in Cement Research, London, England, Vol. 3, No. 10, 1990, pages 85-88.
Gartner, E. M., “The Effects of Minor and Trace Elements on the Manufacture and Use of Portland Cement,” Portland Cement Association, Skokie, Illinois, U.S.A., Internal Report, 1980.
Gartner, E. M., and Tang, F. T., “Formation and Properties of High Sulfate Portland Cement Clinkers,” II Cemneto, Milano, Italy, Vol. 84, April-June 1987, pages 141-164.
Gies, A., and Knöfel, D., “Influence of Alkalis or the Composition of Belite-Rich Cement and the Technological Properties of the Resulting Cements,” Cement and Concrete Research, Elmsford, New York,U.S.A., Vol. 16, No. 3, 1986, pages 411-422.
Gies, A., and Knöfel, D., “Influence of Sulfur on the Composition of Belite-Rich Cement Clinkers and the Technological Properties of the Resulting Cements,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 17, 1987, pages 317-328.
Gilioli, C.; Massazza, F.; and Pezzuoli, M., “Studies on Clinker Calcium Silicates Bearing CaF2 and CaSO4,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 9, 1979, pages 295-302.
Gilioli, C.; Massazza, F.; and Pezzuoli, M., “Relazioni d’Equilibrio nel si Stema CaO-SrO-SiO2,” Il Cemneto, Milano, Italy, Vol. 69, 1972, page 19.
Goswami, G.; Mohapatra, B. N.; and Panda, J. D, “Effect of Fluorosilicate on Cement Raw Mix Burnability and Kiln Build-Up,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 44, 1991, pages 634-637.
Gouda, G. R., “Fluxes Conserve Energy in Cement Manufacture,” Rock Products, Chicago, Illinois, U.S.A., Vol. 83, No. 4., April 1980, pages 52-56.
Greening, N. R.; Copeland, L. E.; and Verbeck, J., Modified Portland Cement and Process, Serial No. 1427, U.S. Patent No. 3,628,973, December 21, 1971.
Gurevich,B.I., and Dobrynina, N. G., “Effect of Calcium Fluoride on the Reaction Capacity of Alumino Silicate Components in the Burning of Phosporous-Anhydride Containing Raw Batches,” Proizvod. Stroit. Mater., Moscow, U.S.S.R., 1977, pages 44-59.
Gutt, W., and Osborne, G. J., “Effect of Manganese, Iron and Fluorine on the Properties of Tricalcium Silicate,” Transactions of British Ceramic Society, Stoke-on-Trent, England, Vol. 68, No. 3, 1969, pages 129-136.
Halicz, L., and Nathan, Y., “The Influence of P2O5 on Clinker Reactions,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 14, No. 1, 1984, pages 11-18.
Hornain, H., “The Distribution of Transition Elements and Their Influences on Some Properties of Clinker and Cement,” Revue des Materiaux de Construction, Lafayette, Paris, France, No. 671-72, August/September 1971, pages 203-218.
Imlach, J. A., “The Influence of Heating Conditions on the Production of Fluorine Containing Portland Cement Clinker,” Cement Technology, London, U.K., Vol. 5, 1974, pages 403-406.
Imlach, J. A., “Assessment of the Role of Chromium in Portland Cement Manufacture,” American Ceramic Society Bulletin, Columbus, Ohio, U.S.A., Vol. 54, 1975, pages 519-522.
Ivashchenko, S. I., “Technology of Modified Cements With Reduced Energy Consumption and Improved Properties,” All-union scientific-technical meeting on the chemistry and technology of cements, Moscow, Parts I and II, 1991, pages 43-46.
Jantzen, C. M., and Glasser, F. P., “Stabilization of Nuclear Waste Constituents in Portland Cement,” American Ceramic Society Bulletin, Columbus, Ohio, U.S.A., Vol. 58 (4), April 1979, pages 459-463.
Jantzen, C. M.; Glasser, F. P.; and Lachowski, “Solid-State Reactions of Sintered Commercial Radwaste Ceramics: I. PUREX and MAGNOX Waste Without Additives,” Material Research Bulletin, Elmsford, New York, U.S.A., Vol. 17, No. 1, 1982, pages 77-87.
Jawed, I., and Skalny, J., “Alkalies in Cement: A review, Part I – Forms of Alkalies and Their Effect on Clinker Formation,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 7, No. 6, 1977, pages 719-730.
Jawed, I., and Skalny, J., “Alkalies in Cement: A review, Part II – Effects of Alkalies on Hydration and Performance of Portland Cement,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 8, No. 1, 1978, pages 37-52.
Johansen, V., Manuscript of Preparation in Madrid, Spain, November, 1977.
Johansen, V., “Solid Solution of Chromium in Ca3SiO5,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vo. 2, No. 1, January 1972, pages 33-42.
Kakali, G.; Kasselouri, V.; and Parrissakis, G., “Hydration and Strength Development of Cements Produced From Raw Mixes Containing MoO3,Nb2O3,WO3 and Zr2O3,” Cement and Concrete Research, Elmsford, New York, U.S.A.,Vol. 19, 1989, pages 968-972.
Kakali, G.; Kasselouri, V.; and Parissakis, G., “Investigation of the Effect of Mo, Nb, W and Zr Oxides on the Formation of Portland Cement Clinker,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 20, 1990, pages 131-138.
Kantro, D. L., “Tricalcium Silicate Hydration in the Presence of Various Salts,” Journal of Testing and Evaluation, Philadelphia, Pennsylvania, U.S.A., Vol. 3, No. 4, July 1975, page 312.
Klein, D. M.; Andren, A. W.; Carter, J. A.; Emery, J. F.; Feldman, C.; Fulkerson, W.; Lyons, W. S.; Ogle, J. C.; Talm, Y.; Van Hook, R. I.; and Bolton, N., “Pathways of Thirty-Seven Trace Elements Through a Coal-Fired Power Plant,” Environmental Science and Technology, Washington, D.C.,
U.S.A., Vol. 9, No. 10, October 1975, pages 973-979.
Klemm, W. A., “Hexavalent Chromium in Portland Cement,” A Report to ASTM Subcommittee C01.10 on Portland Cement, Miami, Florida, U.S.A., December 10, 1992.
Klemm, W. A., and Skalny, J. P., “Mineralizers and Fluxes in Clinkering Process,” Cement Research Progress: Cements Division, American Ceramic Society, Westerville, Ohio, U.S.A., Chapter 14, 1976, pages 259-292.
Klemm, W. A.; Jawed, I.; and Holub, K. J., “Effects of Calcium Fluoride Mineralization on Silicates and Melt Formation on Portland Cement Clinker,” Cement and Concrete Research, Elmsford, New York,U.S.A., Vol. 9, July 1979, pages 489-496.
Knöfel, D., “Modifying Some Properties of Portland Cement Clinker and Portland Cement by Means of TiO2,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, No. 4, 1977, pages 191-196.
Knöfel, D., and Gies, A., “Effect of Manganese on the Properties of Portland Cement Clinker and Portland Cement,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, No.7,1983, pages 402-408.
Knöfel, D.; Strunge, J.; and Bambauer, H. U.,”Incorporation of Manganese in Tricalcium Silicate,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 12, 1984,
pages 651-655.
Knöfel, D., “Modifying Some Properties of Portland Cement Clinker and Portland Cement by Means of ZnO and ZnS,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 31, No. 3, March 1978, pages 157-161.
Knöfel, D., “The Incorporation of TiO2 Into the Phases of Portland Cement Clinker,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 32, No. 1, January 1979, pages 35-40.
Kolovos, K.; Tsivilis, S.; and Kakali, G., “The effect of Foreign Ions on the Reactivity of the CaO-SiO2-Al2O3-Fe2O3 System, Part II: Cations,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 32, 2002, pages 463-469.
Kondo, R., “Effects of Special Components on the Mineral Compositions of Portland Cement Clinker,” Semento Gijutsu Nenpo, Tokyo, Japan, Vol. 17, 1963, pages 42-49.
Kondo, R., and Yoshida, K., “Miscibilities of Special Elements in Tricalcium Silicate and Alite and the Hydration Properties of C3S Solid Solutions,” 5th. International Symposium on Chemistry of Cement, Tokyo, Japan, Vol. I, No. 23, 1968, page 262.
Kravchenko, I. V.; Aleshina, O. K.; and Grikevich, L. N., “Barium Containing Portland Cement,” Trudy Gos. Vses. Nauch.-Issed. Inst. Tsem. Prom., Moscow, U.S.S.R., No. 23, 1970, pages 3-13.
Kurdowski, W., and Szummzr, A., “l’Influence des Composants Minuers sur les Proprietes Hydrauliques du Clinker Portland,” Silicates Industriels, Mons, Belgium, Vol. 33, 1968, page 183.
Kurdowski, W., “Influence of Minor Components on Hydraulic Activity of Portland Cement Clinker,” 6th. International Congress of Chemistry of Cement, Moscow, U.S.S.R., Supplementary Paper 4-5 to Principal Paper I-4, 1974.
Lea, F. M., Chemistry of Cement and Concrete, 3rd. Ed., Arnold, Chemical Publishing Co., New York,U.S.A., and London, U.K., 1971.
Lee, R. E., and von Lehmden, D. J., “Trace Metal Pollution in the Environment,” Air Pollution Control Association Journal, Pittsburgh, Pennsylvania, U.S.A., Vol. 23, No. 10, October 1973,
pages 853-857.
Lokot. A. A., and Azelitskaya, A. D., Trans. Novocherkassk Politech. Inst., Novocherkassk, U.S.S.R., Vol. 189, 1969, page 103.
Long, G. R., “Microstructure and Chemistry of Unhydrated Cements,” Philosophical Transactions of Royal Society, London, U.K., A310, 1983, page 4351.
Lowes, T. M., and Evans, L. P., “Optimization of the Design and Operation of Coal Flames and Cement Kilns,” Journal of the Institute of Energy, 1989, pages 220-229.
Mantus, E. K.; Kelly, K. E.; and Pascoe, G. A., “All Fired Up: Burning Hazardous Waste in Cement Kiln,” Environmental Toxicology International, and the Combustion Research Institute, Seattle, Washington, U.S.A., 1992.
Marinho, M. B., and Glasser, F. P., “Polymorphism and Phase Changes in the Ferrite Phase of Cements Induced by Titanium Substitution,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 14, 1984, pages 360-368.
Minor Elements in Cement Manufacturing
Matkovic, B., and Young, J. F., “Dicalcium Silicates Doped With Phosphates”, 8th. International Congress of Chemistry of Cement, Rio de Jenairo, Brazil, Vol. II, 1986, pages 276-281.
Miller, F. M., “Minor Elements in Cement Clinker,” Paper No. 16, PCA Cement Chemist’s Seminar, Portland Cement Association, Skokie, Illinois, U.S.A., 1976.
Miller, F. M., and Egeløv, A. H., “Relationship Between Cement Kiln Operation and Content of NOx in Kiln Exit Gases,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, No. 33, June 1980, pages 310-313.
Mircea, S., “Decomposition of Tricalcium Silicate With Boric Oxide,” Silikaty, Prauge, Czechoslovakia, Vol. 9, No. 1, 1965, pages 34-42.
Moir, G. K., and Glasser, F. P., “Mineralizer, Modifiers and Activators in the Clinkering Process,” 9th. International Congress of Chemistry of Cement, Delhi, India, Vol. 1, 1992, pages 125-152.
Moir, G. K., “Improvements in the Early Strength Properties of Portland Cement,” Philosophical Transactions of Royal Society, London, U.K., A: 310, 1983, pages 127-138.
Nielsen, P. B., “SO2 and NOx Emissions From Modern Cement Kilns With a View to Future Regulations,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 9, 1991, pages 449-456.
Norbom, H. R., “Application of Suspension Preheater Kilns Versus Other Kilns in North America,” Proceedings of the IEEE, Cement Industry Technical Conference, Miami, Florida, U.S.A., May 1973, 23 pages.
Nurse, R. W., “The Effect of Phosphate on the Constitution and Hardening of Portland Cement,” Journal of Applied Chemistry, Vol. 2 (12), 1952, page 708.
Odler, I., and Abdul-Maula, S., “Effect of Mineralizers on the Burning Process of Portland Cement Clinker, Part 1: Kinetics of the Process,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, No. 3, 1980-a, pages 132-136.
Odler, I., and Abdul-Maula, S.,”Effect of Mineralizers on the Burning Process of Portland Cement Clinker, Part 1: Mode of Action of the Mineralizers,” Zement-Kalk-Gips, Bauverlag
GMBH/Maclean Hunter, Wiesbaden, Germany, No. 6, 1980-b, pages 278-282.
Odler, I., and Schmidt, O., “Structure and Properties of Portland Cement Clinker Doped with Zinc Oxide,” Journal of American Ceramic Society, Columbus, Ohio, U.S.A., 1980-c, pages 13-16.
Palomo, A.; Vásquez, T.; Blanco-Varela, M. T.; and Puertas, F., “The Mineralizer Effect of Fluorspar in Relation to the Form of Addition to an Industrial Raw Mix,” TIZ Fachberichte, Coburg, Germany, Vol. 109, No. 10, 1985, pages 752-755.
Pérez Méndez, M.; Trviño, Vásquez, F.; Vásquez, Moreno T.; and Madrid, E., “Effect of Fluorine on Extracted Phases From Portland Cement Clinker,” TIZ Facherichte, Coburg, Germany, Vol. 110, No.4,1986, pages 286-291.
Peukert, J., “The Technology of Rapid-Hardening or High-Strength Cements From one Clinker,” 6th. International Congress of Chemistry of Cement, Moscow, U.S.S.R. 1974.
Puertas, F.; Glasser, F. P.; Blanco-Varela, M. T.; and Vaquez, T., “Influence of the Kiln Atmosphere on Manganese Solid Solution in C3S and C2S,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 18, No. 5, 1988, pages 783-788.
Ramankulov, M. R.; Butt, Y. M.; and Timoshev, V. V., “Study of the Properties of Minerals and
Cements Containing CdO and TiO2,” Trudy Mosk. Khim.-Tekhnol. Inst., Moscow, U.S.S.R., Vol. 45, 1964, pages 38-44.
Rangarao, M. V., “Effect of Minor Elements on Formation and Properties of Portland Cement Clinker,” Cement, Bombay, India, Vol. 10, No. 3, April-June 1977, pages 6-13.
Richartz, W., “Effect of the K2O Content and Degree of Sulfatization on the Setting and Hardening of Cement,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 12, 1986, pages 678-687.
Rompps Chemie-Lexikon, Bd. 5, Aufl. 8, Franckh’sche Verlagsbuchhandlung, Stuttgart, Germany, 1987.
Rumjanstsev, P. F., and Skotnikova, Z. I., “Influence of Lanthanum Oxide on the Formation Kinetics and Properties of Cement Clinker,” Cementitious Materials of Siberia and Far East, Nauka, Novosibirsk, U.S.S.R., 1970, pages 112-115.
Rumyantsev, P. F., and Kozlov, G. V., “Effect of CdO and CuO Additives on the Kinetics of Cement Clinker Formation and Its Properties,” Epitoanyag, Budapest, Hungary,Vol. 20, No. 10, October 1968, pages 387-390.
Shame, E. G., and Glasser, F. P., “Stable C3S Solutions Containing Fluorine and Aluminum Made Between 1050°C, and 1250°C,” British Ceramics Transactions and Journal, Stoke-on-Trent, England, Vol. 86, 1987, pages 13-17.
Sichov,M.M., “Problem of Admixtures,” Paper I-94, 5th. International Congress of Chemistry of Cement, Tokyo, 1968.
Sinclair, W., and Groves, G. W., “Transmission Electron Microscopy and X-ray Diffraction of Doped Tricalcium Silicate,” Journal of American Ceramic Society, Columbus, Ohio, Vol. 67, No. 5, 1984, pages 325-330.
Skalny, J. P., and Klemm, W. A., “Alkalis in Clinker: Origin, Chemistry, Effects,” Conference on Alkali-Aggregate Reaction in Concrete, Cape Town, South Africa, No. S252, March 30-April 3, 1981, pages 1-7.
Smith, R. D.; Campbell, J. A.; and Nielson, K. K., “Concentration Dependence Upon Particle Size of Volatilized Elements in Fly Ash,” Environmental Science and Technology, Washington, D.C., U.S.A., Vol. 13, No. 5, May 1979, pages 553-565.
Sprung, S.; Kirchner, G.; and Rechenberg, W., “Reaction of Poorly Volatile Trace Elements in Cement Clinker Burning,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 37, No. 10, October 1984, pages 513-518.
Sprung, S., and Rechenberg, W., “The Reactions of Lead and Zinc in the Burning of Cement Clinker,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 31, No.7,July 1978, pages 327-329.
Sprung, S., and von Seebach, H. M., “Fluorine Balance and Fluorine Emission From Cement Kilns,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 1, 1968, pages 1-8.
Sprung, S., “Technological Problems in Pyro-Processing Cement Clinkers: Cause and Solution,” Translation by Brodek, T. V., Beton-Verlag GmbH, Duesseldorf, Germany, 1985.
Minor Elements in Cement Manufacturing
Stephan, D.; Mallmann, R.; Knöfel, D.; and Härdtl, “High Intakes of Cr, Ni, and Zn in Clinker, Part I. Influence on Burning Process and Formation of Phases,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 29, 1999, pages 1949-1957.
Stephan, D. R.; Knöfel, D.; and Härdtl, “Examination of the Influence of Cr, Ni and Zn on the Manufacture and Use of Cement,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 54, No. 6, 2001, pages 335-346.
Stevula, L., and Petrovic, J., “Hydration of Polymorphic Modifications of C3S,” Cement and Concrete Research, Elmsford, New York, U.S.A., Vol. 11, No. 2, 1981, pages 183-190.
Strunge, J.; Knöfel, D.; and Driezler, I., “Influence of Alkalies and Sulfur on the Properties of Cement, Part I: Effect of SO3 Content on the Cement Properties,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 38, 1985, pages 150-158.
Strunge, J.; Knöfel, D.; and Driezler, I., “Influence of Alkalis and Sulfur on the Properties of Cement,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 39, 1986, pages 389-386.
Subba Rao, V. V., and, Narang, K. C., “Potentials of Making Active Belite Cements With Chromium Oxide as Modifier,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 8, 1987, pages 434-437.
Sychev,M.M., and, Korneev, V. I., “The Effect of Raw Material Admixtures on the Properties of Tricalcium Silicate,” Trudy Gos. Vses. Inst. po Proektir. i. Nauchn.-Issled. Rabotam v Tsementn. Prom., Leningrad, U.S.S.R., Vol. 29, 1964, pages 3-12.
Taylor, H.F.W.,Cement Chemistry, 2nd Edition, Thomas Telford Publishing, London, U.K., 1997, 459 pages.
Timashev, V. V.; Ramankulov, M. R.; and Suleimanov, A. T., Prikl. Teor. Khim., Moscow, U.S.S.R., Vol. 5, 1974, page 3.
Timashev, V. V., “The Kinetics of Clinker Formation: the Structure and Composition of Clinker and its Phases,” 7th. International Congress of Chemistry of Cement, Paris, France, Vol. 1, 1980, page I-3/1.
Toropov, N. A., and Boikova, A. I., “Solid Solutions of Tricalcium Silicate With Yttrtium Oxyorthosilicate,” Doklady Academi Nauk SSSR, New York, U.S.A., Vol. 151, No. 5, 1963, pages 1113-1117.
Toropov, N. A., and Fedorov, N. F., “Stabilization of High Temperature Forms of Dicalcium Silicates by Orthosilicates of Lanthanoids,” Zhurnal Prikladnoi Khimi, Leningrad, U.S.S.R., Vol. 35, No 10, 1962-a, pages 2156-2161.
Toropov, N. A., and Fedorov, N. F., “Solid Solutions of Lanthanum Orthosilicate In Dicalcium Silicate,” Zhurnal Prikladnoi Khimi, Leningrad, U.S.S.R., Vol. 35, No 11, 1962-b, pages 2548-2550.
Tsuboi, T.; Ito, T.; Hokinoue, Y.; and Matsuzaki, Y., “The Effects of MgO, SO3, and ZrO on the Sintering of Portland Cement Clinker,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, No. 9, 1972, paAges 426-431.
von Seebach, M., and Tompkins, J. B., “The Behavior of Metals in Cement Kilns,” Rock Products’ 26th International Cement Seminar, New Orleans, December 5, 1990.
Weisweiler, W., and Krˇcmar, W., “Arsenic and Antimony Balances of a Cement Kiln Plant With Grate Preheater,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 3, 1989, pages 133-135.
Weisweiler, W., and Krˇcmar, W., “Heavy Metal Balances of a Cement Kiln Plant With Grate Preheater,” Zement-Kalk-Gips, Bauverlag GMBH/Maclean Hunter, Wiesbaden, Germany, Vol. 3, 1990, pages 149-152.