Contents
Clinker Manufacture – Formation of Clinker
[wpecpp name=”package + Updates forever” price=”250″ align=”center”]
1. Introduction
Why study clinker burning?
“To understand the influence of changes in kiln operation conditions”
-Normal kiln operation
.Influence of chemistry, fineness, mineralogy changes
.Influence of new mix components (pozzolan, AFR, sand, etc.)
-Abnormal kiln operation
.know causes of badly burnt clinker
.understand why rings and deposits form
.be able to suggest counter measure
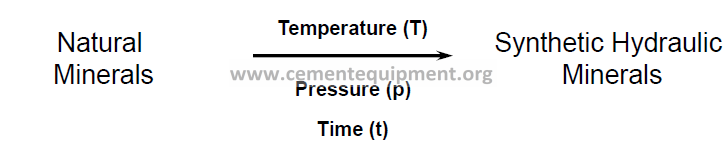
Analogy to transformation of igneous and sedimentary rocks
into metamorphic rocks
Difference in T, p, t
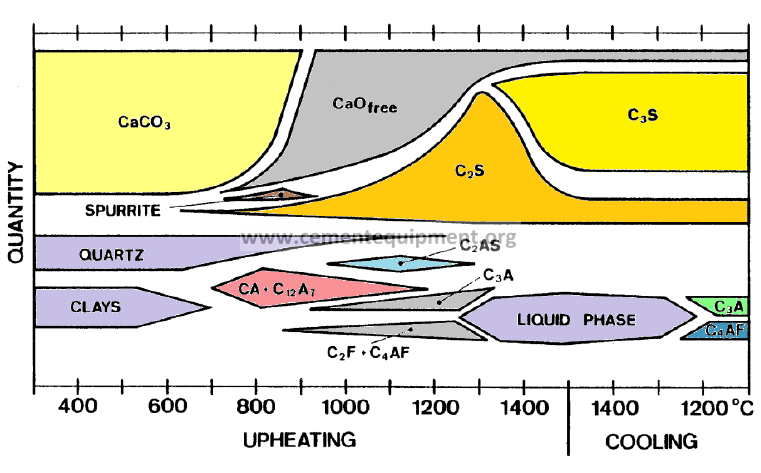
Two principal steps during transformation into clinker
Disintegration of original structure
-Mechanical crushing and grinding
-Thermal decomposition
-Structural rearrangement on heating (e.g. polymorphism)
-melt
Formation of new structures
-Occurrence of intermediate products
-Genesis and growth of final clinker minerals
-Crystallisation of liquid phase
Features characterising the clinker formation process
-Complex system (series of diverse mechanisms)!
-Requires mechanical, thermal and electrical energy
-Reaction rate is slow (necessity of high temperatures, finely dispersed material)
-Clinker minerals are not stable at normal temperature!
Quality of product is determined by:
-Clinker chemistry
-Clinker microstructure
Control of burning process
Material technological aspects
-Raw meal burning behaviour
– Burnability
– Dust formation
– Coating behaviour
– Granulation of clinker
– etc.
-Quantity and properties of liquid phase
Process technological aspects
-Temperature profile
-Kiln atmosphere
-Fuel type
-Flame characteristics
etc.
Aspect
-Reaction pathway
-Reaction mechanism
-Reaction kinetics
-Reaction thermodynamics
Characteristic feature
-indicates the intermediate products occurring between reactants and products
-type(s) and reaction(s) taking place
-indicates rate at which the final products are produced
-dictates whether reaction will be at all possible, and what the heat and temperature requirements will be
Overview
1. Introduction
2. Reaction Pathways Encountered During Clinker Formation
-Basic Sequence of Reactions
-Mineralogical and Chemical Characteristics of Raw Mixes
-intermediate Products
-Liquid Phase
-The Overall Reaction Sequence
3. Reaction Mechanisms
4. Kinetics of Clinker Burning
-Practical Considerations
-Assessment of Raw Meal Burnability
5. Thermodynamics of Clinker Formation
2. Reaction Pathways
To fully describe the pathway of clinkering, it is
necessary to consider the following aspects:
-the chemical and mineralogical content of the raw mix
-The overall sequence of reactions
-the chemical and mineralogical nature of the intermediate products
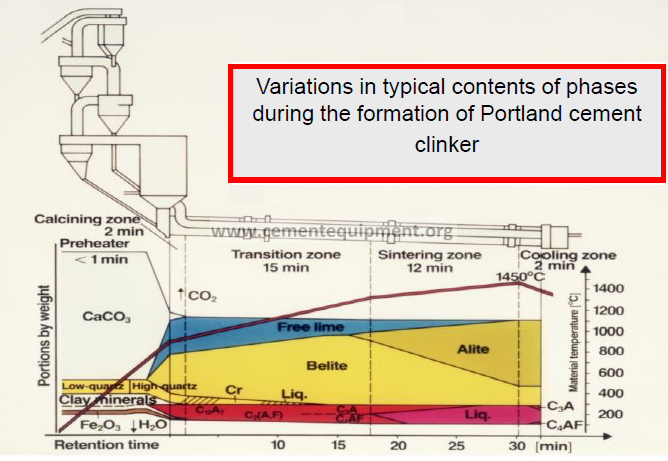
Sequence of reactions occurring in a rotary kiln
Heating (°C)
20 – 100 Evaporation of H2O
100 – 300 Loss of physically adsorbed water
400 – 900 Removal of structural H2O (H2O and OH groups) from clay minerals
>500 Structural changes in silicate minerals
600 – 900 Dissociation of carbonates
>800 Formation of belite, intermediate products, aluminate and ferrite
>1250 Formation of liquid phase (aluminate and ferrite melt)
~1450 Completion of reaction and re-crystallization of alite and belite
Cooling (°C)
1300 – 1240 Crystallization of liquid phase into mainly aluminate and ferrite
Typical chemical characteristics of raw mixes
Parameter X mean X min X max
L.o.I. 35.5 33.8 37.3
SiO2 14.4 12.8 16.0
Al2O3 3.2 2.4 4.6
Fe2O3 1.8 1.0 3.8
CaO 42.4 39.0 44.2
SO3 0.37 0.08 1.1
Na2O 0.17 0.04 0.58
LS 94.0 85.4 103.4
SR 2.9 1.8 3.9
AR 1.9 0.7 3.2
Intermediate products encountered during clinker production
Type Mineral Name Formula
Simple Sulfates anhydrite CaSO4
arcanite K2SO4
Compound Sulfates “sulfate”-spurrite 2(C2S) . Ca SO4
“calcium”-langbeinite K2Ca2 (SO4)3
Compound Carbonates spurrite 2(C2S) . CaCO3
Simple Chlorides sylvite KCl
Calcium Aluminates mayenite 12 CaO . 7 Al2O3
CaO .Al2O3
Calcium Ferrites 2 CaO.Fe2O
Calcium Alumino-Silicates gehlenite 2 CaO.Al2O3. SiO2
Reasons for the formation of intermediate products
-Intermediate products are preferentially formed by kinetically faster reaction rates
-Intermediate products are the reaction products of localised zones in the meal charge, i.e. local equilibrium but not overall equilibrium was reached (e.g. gehlenite formation)
-Intermediate products are really the equilibrium products at the given temperature and gas atmosphere, but not at the final clinkering temperature (e.g. spurrite formation)
Liquid Phase
basically created by early melting compounds such as Fe2O3 and Al2O3 and some minor compounds such as MgO and Alkalis
The composition of the raw mix determines
-temperature at which liquid will first be formed
-amount of liquid formed at any given temperature
-the physical properties of the liquid at any particular temperature, especially its viscosity
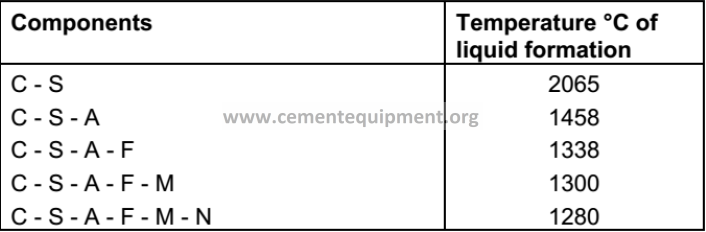
Temperature of Liquid Formation
-The following are the lowest melting temperatures of various mixtures pertinent to Portland cement production:
-Due to the inclusion of additional oxides (e.g. K2O, TiO2, Mn2O3, etc.) the lowest formation temperature of the liquid phase in clinker is in reality around 1250°C.
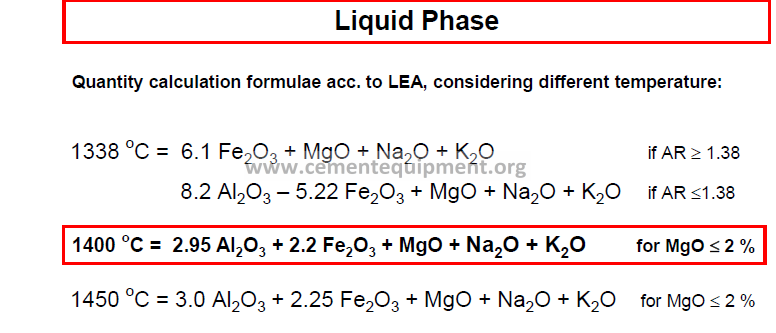
Liquid Phase Quantity of Liquid
-Although most raw mixes show about the same minimum temperature of liquid formation (eutectic point), the quantity of liquid formed at this and progressively higher temperatures varies according to the raw mix chemistry.
-In the Portland cement relevant parts of the system C – S – A – F, in which melting begins at 1338 °C, the composition of the liquid is:
CaO – 55
SiO2 – 6 % Alumina ratio
Al2O3 – 23 % (AR) = 1.38
Fe2O3 – 16 %
Quantitative change of liquid phase with temperature in several group plants (influence of
MgO, Na2O
and K2O
included)
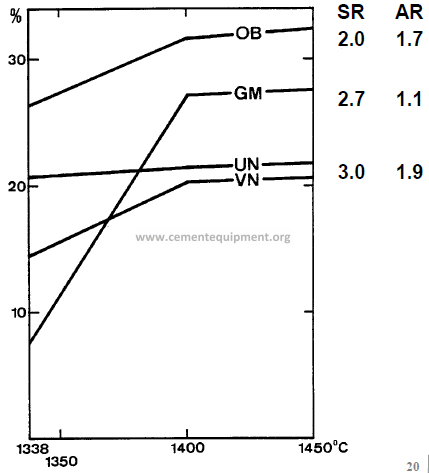
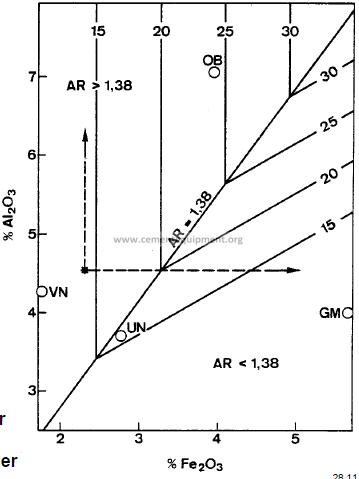
displays graphically the influence of Al2O3and Fe2O3 alone on the quantity of liquid formed at 1338 °C, the solid contours representing the percentage of liquid formed. It can be seen that the effect of adding Fe2O3or Al2O3on the proportion of liquid formed at 1338 °C is influenced strongly by the alumina ratio. in a clinker composition containing 4.5 % Al2O3 and 2 % Fe2O3, for example, the Fe2O3 content is held constant and the Al2O3 content is increased, the percentage of liquid remains unchanged at 14 %, because the iso-Fe2O3 contour lies parallel to the iso-liquid contour in this region.
If, however, the iron content is increased and the Al2O3content remains constant, the proportion of liquid at first increases
rapidly until at 3.3 % Fe2O3 it attains a maximum of
21 %. With further increase in Fe2O3the quantity of liquid decreases.
Therefore, the most effective use of Al2O3and Fe2O3- with respect to liquid formation at 1338 °C – occurs when the two are used in the weight ratio of 1.38.
Influence of Al2O3 and Fe2O3 alone on the quantity of liquid formed at 1338 °C. The most effective use of Al2O3 and Fe2O3 – with respect to liquid formation at 1338 °C – occurs when the two are used in the weight ratio of 1.38
AR = Alumina ratio OB, GM, UN, VN = Different Plants At 1400 °C and above increasing Al2O3 and / or Fe2O3always result in a larger quantity of liquid phase, Al2O3 being the one with the larger influence.
Overview
1. Introduction
2. Reaction Pathways Encountered During Clinker Formation
-Basic Sequence of Reactions
-Mineralogical and Chemical Characteristics of Raw Mixes
-Intermediate Products
-Liquid Phase
-The Overall Reaction Sequence
3. Reaction Mechanisms
4. Kinetics of Clinker Burning
-Practical Considerations
-Assessment of Raw Meal Burnability
5. Thermodynamics of Clinker Formation
3. Reaction Mechanisms – Definitions
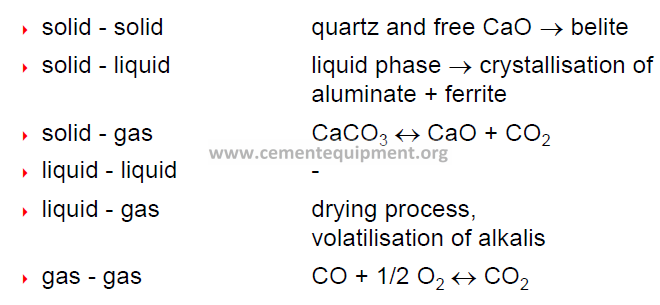
Classification of reactions
2. according to the state of matter:
-State of matter
-solid definitive volume and definite shape
-liquid definitive volume, assumes shape of container
-gaseous neither definitive volume nor definite shape
Classification of reactions
1. according to their type:
3- according to rate controlling step (kinetics of reaction)

3. Reaction Mechanisms – Examples
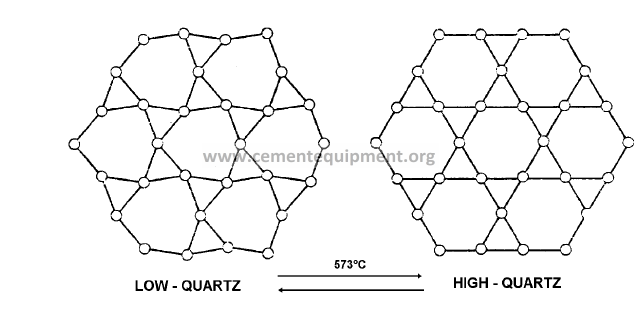
Structural changes: Arrangement of the atoms in low and high quartz
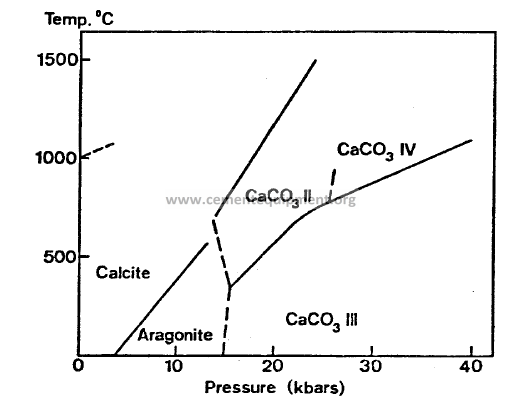
Structural changes: Calcite – Aragonite transition
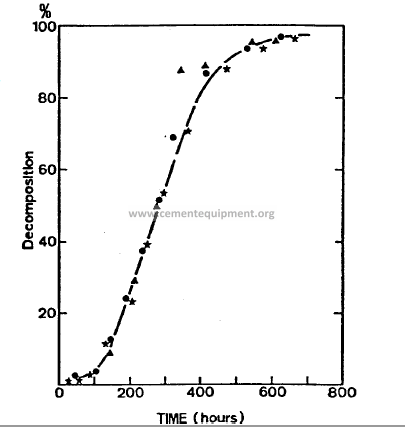
Decomposition reactions (during clinker production)
solid / gas type
-De-hydroxylation of the clay minerals (kaolinite, etc.)
-De-carbonation of the carbonate minerals (magnesite, dolomite, calcite, spurrite)
solid / solid type
-decomposition of alite
Characteristic of this reaction type is that the single reactant is transformed into two products.
Decomposition reaction: Decomposition of C3S at 1175 °C
In the case of impure C3S, i.e. clinker alite, the rate of decomposition is appreciably accelerated by:
• the presence of lime and C2S nuclei
• the presence of Fe2+ , H2O and K2SO4 / CaSO4 melts
Combination reaction: Formation of Alite
Formation of alite only at T > 1250 °C (lower stability limit). At that temperature, the liquid phase is also starting to form: The formation of alite is a liquid – solid reaction.
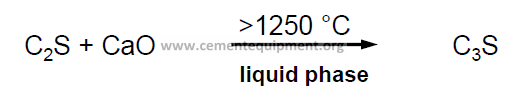
The formation of alite and its stabilisation depends on the presence of the liquid phase. The rate of reaction is dependent on:
-the path distance that the diffusing species have to travel
– quantity and viscosity of liquid phase
Overview
1. Introduction
2. Reaction Pathways Encountered During Clinker Formation
-basic Sequence of Reactions
-Mineralogical and Chemical Characteristics of Raw Mixes
-intermediate Products
-Liquid Phase
-The Overall Reaction Sequence
3. Reaction Mechanisms
4. Kinetics of Clinker Burning
-Practical Considerations
-Assessment of Raw Meal Burnability
5. Thermodynamics of Clinker Formation
4. Kinetics of Clinker Burning
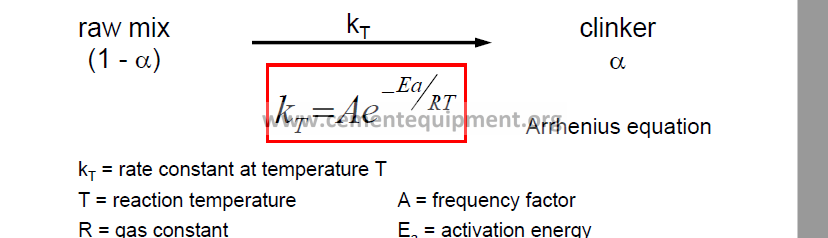
Theoretical consequences:
– Rate increases with higher temperature (but also costs!)
– Rate decreases with higher activation energy
(different raw mix mineralogy)
– Rate increases with higher frequency factor
(larger contact surface, i.e. finer mix)
The rate of reaction
-increases with temperature and contact surface between raw mix components (frequency factor A)
-decreases with higher activation energy Ea for raw mix components.
To compensate for the slow reactivity of the less reactive minerals, a higher burning temperature and / or longer burning period (longer clinkering zone) is required
-increases with temperature and contact surface between raw mix components (frequency factor A)
-decreases with higher activation energy Ea for raw mix components.
To compensate for the slow reactivity of the less reactive minerals, a higher burning temperature and / or longer burning period (longer clinkering zone) is required
-It is evident that the difference in mineralogy and actual particle size of the individual crystals influence both the mechanism and rate of reaction, especially at start of the clinker formation

Assessment of Raw Meal Burnability
-In practice, simple methods are mostly applied to asses the “burnability” of a mix, i.e. the ease of formation of the clinker minerals. Three distinct methods are practiced at HGRS:
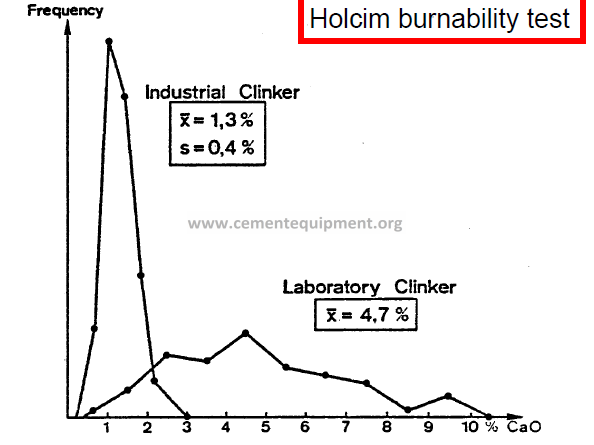
-Holcim burnability test – involves the heating of a raw mix under well defined laboratory conditions, and subsequent laboratory determination of the non-combined lime.
-Statistical burning model – in which ten material parameters influence the rate of clinker formation. The non-combined CaO value, of any raw mix, relative to that of a standard raw mix is calculated.
-Physicochemical burning model – requires no standard raw mix. Only 4 parameters need to be considered
burnability test
-The test is based on the isothermal burning at 1400 °C of raw meal nodules
-The test allows the determination of the relative influence of the various material parameters to be ascertained, free from the influence of process technological disturbances.
Evaluation Free Lime %
very good 0 – 2
goo d 2 – 4
moderate 4 – 6
poor 6 – 8
very poor > 8
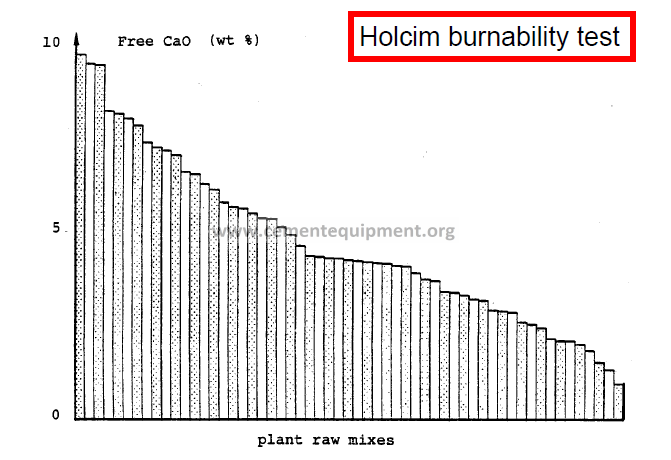
Statistical Burnability Model
-Quantitative evaluation of the data obtained by the Holcim burnability test
-The 1350, 1400 and 1450 free lime values of other raw mixes from the same raw material components can be determined based on one single burnability test of one mix
-Chemical Parameters: lime saturation, silica ratio, alumina ratio, K2O + Na2O, MgO
-Physical Parameters: residue on 200 m and 90 m sieves, quantity of mica, quartz and iron minerals
The burnability model can be used as an
instrument for optimization of raw mixes
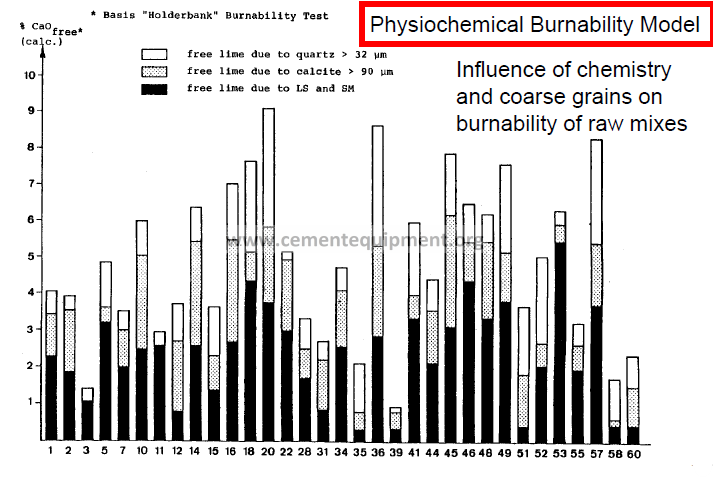
Physiochemical Burnability Model
the amount of uncombined lime depends on
Specific reaction area (area of contact between grains)
Local oversaturation (grain size of individual minerals)
Ambient conditions (pressure, temperature, burning time)
Diffusion coefficient of CaO through the liquid phase (composition of the liquid phase)
Amount of liquid phase formed during burning
Supply and demand of CaO
all these influencing factors may be incorporated in four parameters: SR, LS, amount of oversized quartz grains, amount of oversized calcite grains. (Pressure, temperature and burning time are considered to be constant.)
Silica ratio (SR) and lime saturation (LS)
The formation of C3S from C2S and CaO is governed by the diffusion of CaO through the melt. The silica modules and lime saturation are sufficient to describe this chemical reaction quantitatively.
The amount of CaO which can be accommodated within the liquid phase and in which it can diffuse and thus react, is inversely proportional to the silica ratio. A linear relationship exists between max. lime saturation and silica ratio values at which no free lime can be observed.
Quartz and calcite grains
Whether a grain of material reacts fully under given burning conditions depends on its diameter, structure and chemical composition.
Too large calcite grains result in CaO not being completely combined as also results from grains whose lime saturation is over 100 %.
For the “Holderbank” burnability test conditions the following grain diameters were found to be critical limits:
quartz > 32 um
calcite > 90 um
Overview
1. Introduction
2. Reaction Pathways Encountered During Clinker Formation
Basic Sequence of Reactions
Mineralogical and Chemical Characteristics of Raw Mixes
Intermediate Products
Liquid Phase
The Overall Reaction Sequence
3. Reaction Mechanisms
4. Kinetics of Clinker Burning
Practical Considerations
Assessment of Raw Meal Burnability
5. Thermodynamics of Clinker Formation
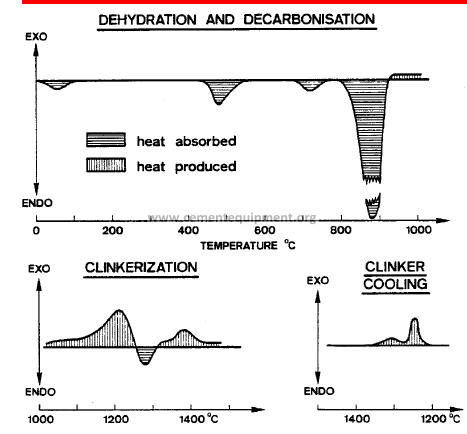
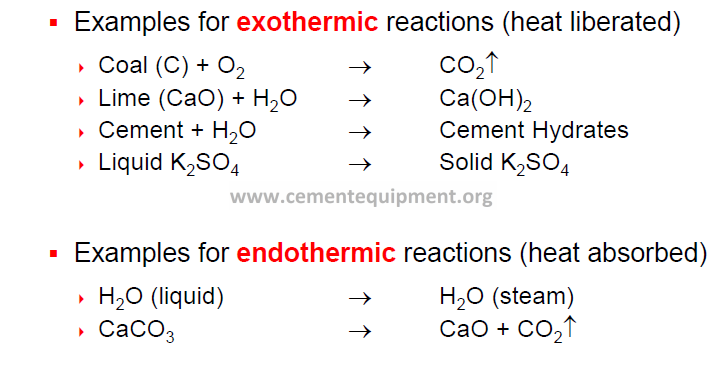
During clinker production, heat is both absorbed (endothermic heat changes) and produced (exothermic heat changes).
Temp. (°C) Type of Reaction Heat Change
20 – 100 Evaporation of free H2 O Endothermic
100 – 300 Loss of physically adsorbed H2O Endothermic
400 – 900 Removal of structural H2O (H2O, OH groups from clay minerals) Endothermic
600 – 900 Dissociation of CO2 from carbonate Endothermic
> 800 Formation of intermediate products, belite, aluminate and ferrite Exothermic
> 1250 Formation of liquid phase (aluminate and ferrite melt) Endothermic
Formation of alite Exothermic
1300 – 1240 Crystallization of liquid phase into mainly (cooling cycle) aluminate and ferrite Exothermic
DTA curves of typical cement raw meals
The greatest heat requirement occurs between 850 – 900 °C, i.e. for the decomposition of the carbonate minerals.The total heat requirements for dehydration, decarbonisation and melting exceed the heat liberated by the formation of belite and the intermediate and final products.