Contents
Cement kiln energy efficiency and productivity Cement Kiln Feed Burnability
TO DOWNLOAD THIS POST AND ALL OTHER IMPORTANT BOOKS IN CEMENT INDUSTRY KINDLY CLICK HERE
In the introduction we saw that the energy consumed by the chemical reactions in the cement kiln can vary from 1630~1840 kJ/kg clinker (390~440 kcal/kg). Explanation of this variation in the “burnability” of the kiln feed is the subject of this session.
Must remember that this energy consumption is the aggregate energy consumption (and liberation) of a sequence of reactions. Evaporation of any residual water in the kiln feed, dehydration of clay minerals, CaCO3 calcination, clinker flux formation and final combination of CaO and C2S to form C3S.
The evaporation of any residual water, dehydration of clay minerals, CaCO3 calcination and clinker flux formation are all endothermic and consume large amounts of energy.
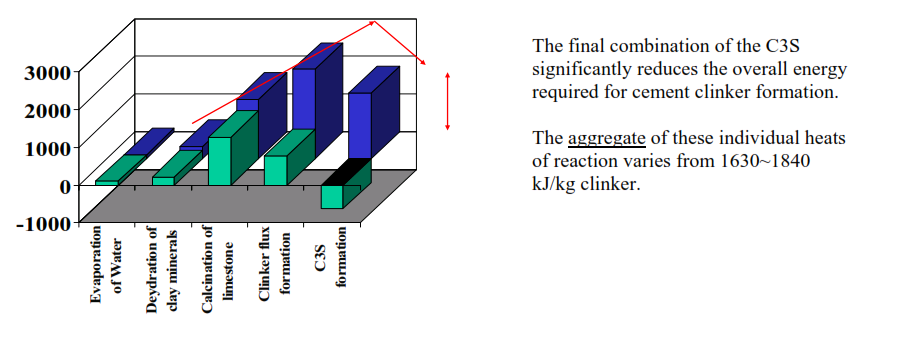
Measuring the energy consumed or liberated by these individual reactions by calorimeter is difficult. A relative measure of the “burnability” of a kiln feed can be obtained in the laboratory by measuring the residual free CaO content after heating a compacted sample to 1400°C for 30 minutes.
Higher residual free CaO indicates a kiln feed with a lower “burnability”, i.e. one that is more difficult to burn to complete combination of the lime with the acidic oxides SiO2, Al2O3 and Fe2O3. However, this does not provide a measure of the kJ/kg of energy which will be consumed in manufacturing clinker. Modelling the aggregate energy consumption based on the chemical and mineral composition of the kiln feed is a means to make this prediction.
The following formula for the assessment of the energy of clinker formation is based on the work of H. zur Strassen:
kJ/kg = 3200xCaO + 2710xMgO – 2140xSiO2 – 250xFe2O3 + Residual Energy
CaO and MgO increase the energy consumption of clinker manufacture, while SiO2 and Fe2O3 reduce the energy consumption (all based on the clinker oxide composition). I.e. increasing lime saturation increases thermal energy consumption. The highly endothermic (energy consuming) calcination reaction of CaCO3 and MgCO3 explains the increases in energy consumption with higher content of these oxides.
The Residual Energy depends on the Al2O3 content of the clinker, the combined (hydrate) water content and the type of clay component in the kiln feed. This combined water content depends on whether the clay component consists of the clay minerals kaolinite, montmorillonite or illite. If the amount of hydrated water and the type of clay is not known then the Residual Energy is calculated by:
Residual Energy = 1720xAl2O3
I.e. Al2O3 content increases thermal energy consumption. If the amount of hydrated water is known, but not the type of clay then the Residual Energy is calculated by:
Residual Energy = 120xAl2O3 + 5520xH2O
If the type of clay is known, but not the amount of hydrated water then the Residual Energy is calculated by:
Residual Energy = 2220x(Al2O3)Kaolinite + 1310x(Al2O3)Montmorillonite +1640x(Al2O3)Illite
If the amount of hydrated water and the type of clay are known then the Residual Energy is calculated by:
Residual Energy = 1400x(Al2O3)Kaolinite+ 620x(Al2O3)Montmorillonite+ 760x(Al2O3)Illite+ 2450xH2O
One the most important contributions of this formula is its recognition of the importance of the clay raw material mineralogy in determining the energy consumed in clinker formation. However, a weaknesses is not making any distinction between the types of silica present in the kiln feed. The SiO2 present as quartz in the kiln feed is recognised to have a significant effect on the burnability of the kiln feed.
As the wet process of cement clinker manufacture has been phased out due to its high thermal energy consumption, clay minerals are now less common as the source of SiO2 and Al2O3 in kiln feed raw mix.
H. Zur Strassen’s method presumes that the entire Al2O3 content of the kiln feed derives from kaolinite, montmorillonite and illite.
The importance of the quartz content of the kiln feed is recognised in the F.L. Smidth burnability formula:

Where:
Q45 is the quartz content of the kiln feed residue on a 45μ sieve.
C125 is the calcite content of the kiln feed residue on a 125μ sieve.
R45 is the content of other insoluble minerals in the kiln feed residue on a 45μ sieve.
Of course, this equation does not provide an estimate of the energy consumption of clinker formation, rather it provides a prediction of the relative “burnability” of a kiln feed, i.e. the residual free lime after heating to 1400°C under controlled conditions. However, F.L. Smidth have developed a method of predicting the energy of clinker
formation based on “first principles”. This method is based on the average thermodynamic values of well characterised, common minerals.
The F.L.S. method considers the formation of clinker as taking place in two distinct steps:
- First all the raw minerals are decomposed (oxidised) to pure oxides in their most stable state at 25°C, and gaseous products such as CO2 and H2O.
- Then the pure oxides are combined to form the clinker minerals, alite, belite, etc, and minor constituents of clinker such as MgO and mixed alkali-calcium sulphates.
Thermodynamically it does not matter that pure SiO2 or Al2O3 are never formed in the cement kiln. If specified reactants are converted to specified products, the total heat of reaction or enthalpy change will be the same, irrespective of the sequence of reactions, or intermediate products formed. The exothermic energy release when the clinker minerals are formed in step 2 is accurately known. The endothermic energy consumed when calcium and magnesium carbonate are converted to their constituent oxides in step 1 is also accurately known.
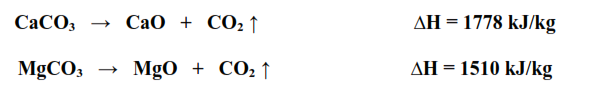
The main source of uncertainty in estimating the heat of clinker formation is in the conversion of non-carbonate components of the raw mix to their pure oxides, i.e. the SiO2 and Al2O3 bearing minerals.
Zur Strassen proposed that the non-carbonate components was made up of a mixture of the kaolinite, montmorillonite and illite clay minerals, but this is often not the case with modern dry process raw mixes, also, analysing the raw mix to quantitatively determine the quantities of these clay minerals present is difficult. The chemical formulae and enthalpies for decomposition of these minerals to pure oxides are not accurately defined.
The major contribution of the FLS methodology is its approach to clarifying this uncertainty as to the energy required for the conversion of the non-carbonate components of the raw mix to their pure oxides. While the enthalpies of formation of kaolinite, montmorillonite and illite may not be precisely known, there are alumino-silicate minerals, in combination with various other oxides (K2O, Na2O, CaO, MgO, etc.) whose enthalpies of formation are well established.
The proposition in the FLS methodology is that the difference between these enthalpies of formation and the enthalpies of formation of the pure oxides is determined by the energy required to remove the other oxides from the acidic silica in the minerals.
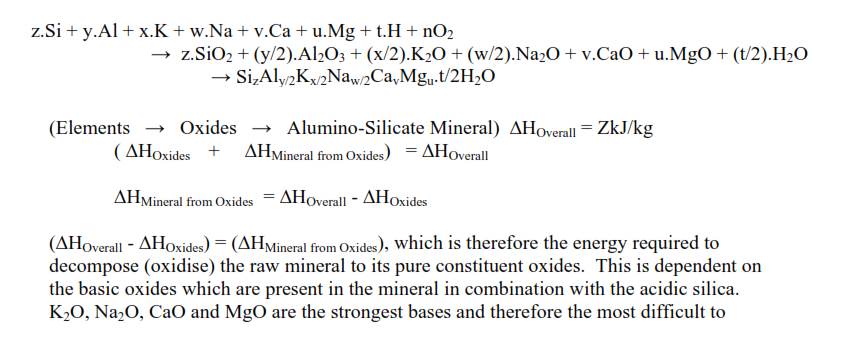
remove from the SiO2 in the mineral, increasing the heat of formation of clinker. Al2O3 and Fe2O3 are much weaker bases and therefore easier to remove from the SiO2 in the raw minerals and have little effect on the energy of clinker formation.
Assessing the energy consumed in the decomposition of the raw minerals starts with the chemical analysis of the raw mix:
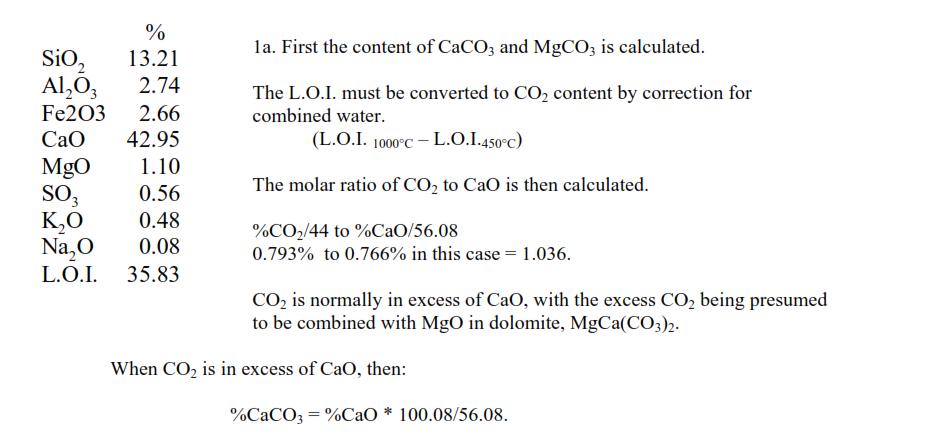
If CaO is in excess of CO2 then the excess CaO is presumed to be combined in the clay minerals and % CaCO3 = %CO2*100.08/44. The molar ratio of the excess CO2 to MgO is then calculated.
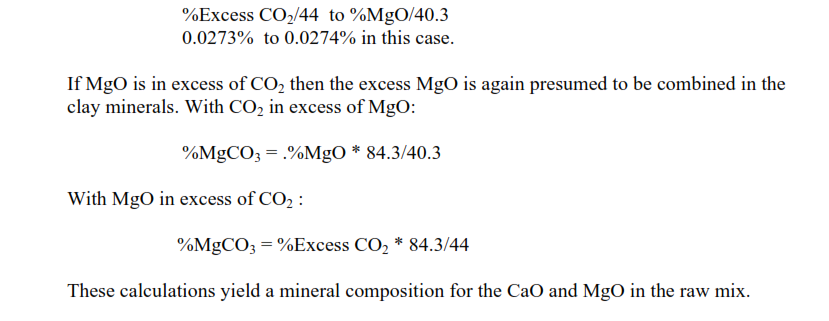
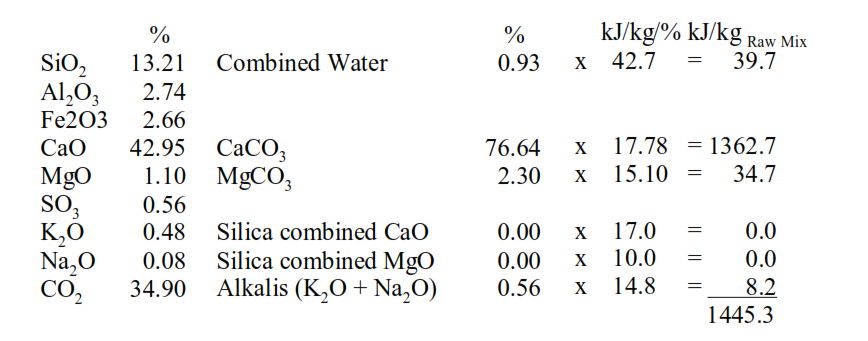
These are the minerals, along with the total alkalis, which consume the energy in the decomposition of the raw minerals. The percentages of each of these minerals are multiplied by their conversion factor in kJ/kg/% to give the heat of decomposition. The thermal energy consumption for clinker formation then depends on the fuel ash contribution to the clinker and the raw mix to clinker factor.
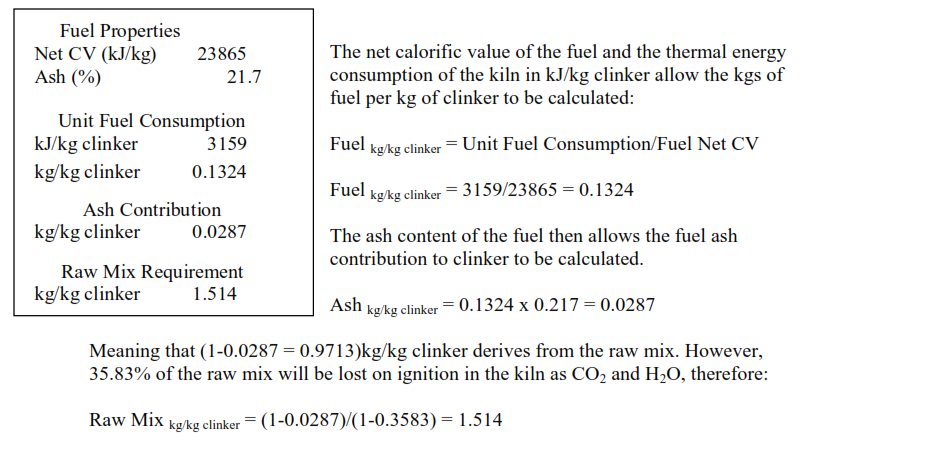
This is the factor which is used to calculate the thermal energy consumption for clinker formation.
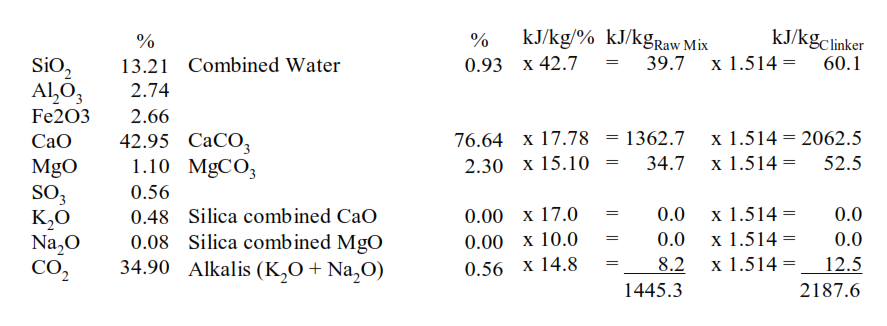
This is the energy required for decomposition of the raw minerals calculated by the FLS methodology. The energy required for step 2, combination of the pure oxides to form the clinker minerals, alite, belite, etc., must be calculated.
First the chemical composition of the clinker must be calculated from the chemical analysis of the raw mix and the contribution of the fuel ash:
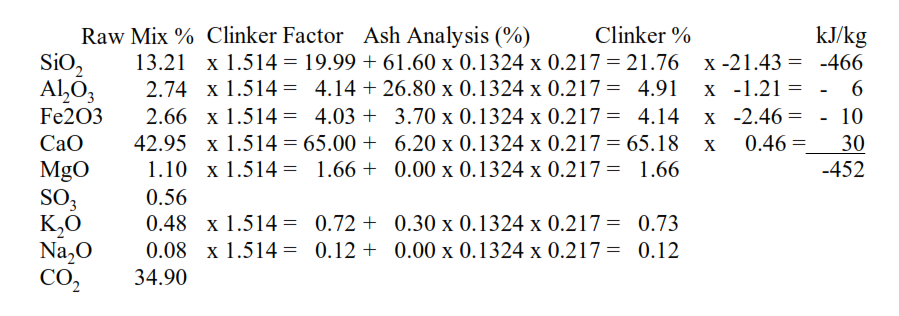
The energy required for clinker mineral formation is then calculated by multiplying the clinker CaO, SiO2, Al2O3 and Fe2O3 contents by coefficients. The coefficients are applied to the oxide composition of the clinker, but the heat evolution is associated with the formation of C2S, C3A and C4AF. The formation of C3S from C2S and CaO absorbs some heat energy, hence the positive coefficient applied to the CaO content.
First energy absorbed in step 1, decomposition of the raw minerals to their oxides, is then added to the heat evolved in step 2, formation of the clinker minerals, to arrive at the overall heat of clinker formation:

This overall energy for clinker formation lies in the middle of the range of 1630~1840 kJ/kg clinker which has been observed in practice. This FLS approach is good because it allows the heat of clinker formation to be calculated from the raw mix composition without needing to identify the exact non-carbonate minerals present. However, it does not explain the observed impact of the SiO2 mineral form on the burnability.
In fact as SiO2 in quartz is not associated with any CaO, MgO, K2O or Na2O the methodology calculates a lower heat of clinker formation if the SiO2 is present as quartz. The impact of quartz on the burnability of raw mix is reduced by finer grinding and changing the granulometry of the raw mix. Thermodynamic considerations alone are insufficient for explaining burnability or heat of clinker formation. Reaction kinetics also have to be taken into consideration, we will discuss these in the next session of the course.
However, before leaving the subject of thermodynamics and the FLS methodology, we need to briefly consider the impact of sulphides and organic components in the raw mix.
In module 3 we will see that the presence of sulphides and organics in the raw mix is unwelcome due to their potential impact on the emissions from the cement kiln.
When both sulphides and organics in the raw mix oxidise, energy is evolved and therefore, the heat of clinker formation is reduced.
Assessing the impact requires knowledge of the sulphate, total sulphur and organic carbon content of the raw mix (i.e. the carbon content excluding that in the carbonates, CaCO3 and MgCO3).
When both sulphides and organics in the raw mix oxidise energy is evolved and therefore the heat of clinker formation is reduced.
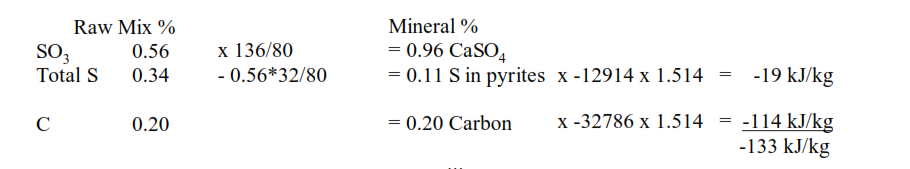
The sulphate is presumed to be present as calcium sulphate, CaSO4, and the excess sulphur presumed to be pyrites, FeS2.
The pyrites, FeS2, will partially oxidise to SO2 in the preheater, with the remainder being oxidised to sulphate and passing out of the kiln in the clinker, with the oxidation yielding thermal energy. As will the organic carbon.
Despite this reduction in the heat of clinker formation the thermal energy consumption of a cement kiln might not be reduced, the oxidation takes place in the top stages of the preheater and therefore the temperature of the kiln exhaust gases is raised. High kiln exhaust gas temperature means more energy losses from the kiln. This illustrates the importance of the location in the process where these reactions take place, again a topic to be covered in subsequent sessions of the course.
TO DOWNLOAD THIS POST AND ALL OTHER IMPORTANT BOOKS IN CEMENT INDUSTRY KINDLY CLICK HERE